PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65
- PMID: 22724072
- PMCID: PMC3376738
- DOI: 10.1098/rsob.120080
PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65
Abstract
Missense mutations in PTEN-induced kinase 1 (PINK1) cause autosomal-recessive inherited Parkinson's disease (PD). We have exploited our recent discovery that recombinant insect PINK1 is catalytically active to test whether PINK1 directly phosphorylates 15 proteins encoded by PD-associated genes as well as proteins reported to bind PINK1. We have discovered that insect PINK1 efficiently phosphorylates only one of these proteins, namely the E3 ligase Parkin. We have mapped the phosphorylation site to a highly conserved residue within the Ubl domain of Parkin at Ser(65). We show that human PINK1 is specifically activated by mitochondrial membrane potential (Δψm) depolarization, enabling it to phosphorylate Parkin at Ser(65). We further show that phosphorylation of Parkin at Ser(65) leads to marked activation of its E3 ligase activity that is prevented by mutation of Ser(65) or inactivation of PINK1. We provide evidence that once activated, PINK1 autophosphorylates at several residues, including Thr(257), which is accompanied by an electrophoretic mobility band-shift. These results provide the first evidence that PINK1 is activated following Δψm depolarization and suggest that PINK1 directly phosphorylates and activates Parkin. Our findings indicate that monitoring phosphorylation of Parkin at Ser(65) and/or PINK1 at Thr(257) represent the first biomarkers for examining activity of the PINK1-Parkin signalling pathway in vivo. Our findings also suggest that small molecule activators of Parkin that mimic the effect of PINK1 phosphorylation may confer therapeutic benefit for PD.
Keywords: PINK1; Parkin; Parkinson's disease.
Figures




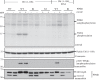

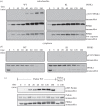

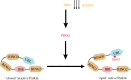
References
-
- Muqit MM, et al. 2006. Altered cleavage and localization of PINK1 to aggresomes in the presence of proteasomal stress. J. Neurochem. 98, 156–169 10.1111/j.1471-4159.2006.03845.x (doi:10.1111/j.1471-4159.2006.03845.x) - DOI - PubMed
-
- Valente EM, et al. 2004. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 10.1126/science.1096284 (doi:10.1126/science.1096284) - DOI - PubMed
-
- Woodroof HI, et al. 2011. Discovery of catalytically active orthologues of the Parkinson's disease kinase PINK1: analysis of substrate specificity and impact of mutations. Open Biol. 1, 110012. 10.1098/rsob.110012 (doi:10.1098/rsob.110012) - DOI - PMC - PubMed
-
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. 2006. Drosophila PINK1 is required for mitochondrial function and interacts genetically with Parkin. Nature 441, 1162–1166 10.1038/nature04779 (doi:10.1038/nature04779) - DOI - PubMed
-
- Park J, et al. 2006. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by Parkin. Nature 441, 1157–1161 10.1038/nature04788 (doi:10.1038/nature04788) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
