Biodegradable optode-based nanosensors for in vivo monitoring
- PMID: 22725692
- PMCID: PMC3683867
- DOI: 10.1021/ac301137c
Biodegradable optode-based nanosensors for in vivo monitoring
Abstract
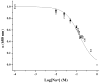
Optode-based fluorescent nanosensors are being developed for monitoring important disease states such as hyponatremia and diabetes. However, traditional optode-based sensors are composed of nonbiodegradable polymers such as poly(vinyl chloride) (PVC) raising toxicity concerns for long-term in vivo use. Here, we report the development of the first biodegradable optode-based nanosensors that maintain sensing characteristics similar to those of traditional optode sensors. The polymer matrix of these sensors is composed of polycaprolactone (PCL) and a citric acid ester plasticizer. The PCL-based nanosensors yielded a dynamic and reversible response to sodium, were tuned to respond to extracellular sodium concentrations, and had a lifetime of at least 14 days at physiological temperature. When in the presence of lipase, the nanosensors degraded within 4 h at lipase concentrations found in the liver but were present after 3 days at lipase concentrations found in serum. The development of biodegradable nanosensors is not only a positive step towards their future use in in vivo applications, but they also represent a new sensor platform that can be extended to other sensing mechanisms.
Figures




References
-
- Seiler K, Wang K, Bakker E, Morf WE, Rusterholz B, Spichiger UE, Simon W. Clin Chem (Washington, DC, US) 1991;37(8):1350–5. - PubMed
-
- Wang K, Seiler K, Morf WE, Spichiger UE, Simon W, Lindner E, Pungor E. Anal Sci. 1990;6(5):715.
-
- Morf WE, Seiler K, Rusterholz B, Simon W. Anal Chem (Washington, DC, US) 1990;62(7):738–742.
-
- Trettnak W, Wolfbeis OS. Anal Chim Acta. 1989;221(0):195–203.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

