Protein arginine methyltransferase 5 is a potential oncoprotein that upregulates G1 cyclins/cyclin-dependent kinases and the phosphoinositide 3-kinase/AKT signaling cascade
- PMID: 22726390
- PMCID: PMC7659304
- DOI: 10.1111/j.1349-7006.2012.02367.x
Protein arginine methyltransferase 5 is a potential oncoprotein that upregulates G1 cyclins/cyclin-dependent kinases and the phosphoinositide 3-kinase/AKT signaling cascade
Abstract
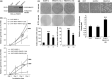
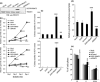
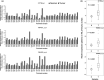
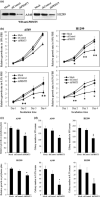
Increasing evidence suggests that PRMT5, a protein arginine methyltransferase, is involved in tumorigenesis. However, no systematic research has demonstrated the cell-transforming activity of PRMT5. We investigated the involvement of PRMT5 in tumor formation. First, we showed that PRMT5 was associated with many human cancers, through statistical analysis of microarray data in the NCBI GEO database. Overexpression of ectopic PRMT5 per se or its specific shRNA enhanced or reduced cell growth under conditions of normal or low concentrations of serum, low cell density, and poor cell attachment. A stable clone that expressed exogenous PRMT5 formed tumors in nude mice, which demonstrated that PRMT5 is a potential oncoprotein. PRMT5 accelerated cell cycle progression through G1 phase and modulated regulators of G1; for example, it upregulated cyclin-dependent kinase (CDK) 4, CDK6, and cyclins D1, D2 and E1, and inactivated retinoblastoma protein (Rb). Moreover, PRMT5 activated phosphoinositide 3-kinase (PI3K)/AKT and suppressed c-Jun N-terminal kinase (JNK)/c-Jun signaling cascades. However, only inhibition of PI3K activity, and not overexpression of JNK, blocked PRMT5-induced cell proliferation. Further analysis of PRMT5 expression in 64 samples of human lung cancer tissues by microarray and western blot analysis revealed a tight association of PRMT5 with lung cancer. Knockdown of PRMT5 retarded cell growth of lung cancer cell lines A549 and H1299. In conclusion, to the best of our knowledge, we have characterized the cell-transforming activity of PRMT5 and delineated its underlying mechanisms for the first time.
© 2012 Japanese Cancer Association.
Figures




References
-
- Krapivinsky G, Pu W, Wickman K, Krapivinsky L, Clapham DE. pICln binds to a mammalian homolog of a yeast protein involved in regulation of cell morphology. J Biol Chem 1998; 273: 10811–4. - PubMed
-
- Pollack BP, Kotenko SV, He W, Izotova LS, Barnoski BL, Pestka S. The human homologue of the yeast proteins Skb1 and Hsl7p interacts with Jak kinases and contains protein methyltransferase activity. J Biol Chem 1999; 274: 31531–42. - PubMed
-
- Lee JH, Cook JR, Pollack BP, Kinzy TG, Norris D, Pestka S. Hsl7p, the yeast homologue of human JBP1, is a protein methyltransferase. Biochem Biophys Res Commun 2000; 274: 105–11. - PubMed
-
- Rho J, Choi S, Seong YR, Cho WK, Kim SH, Im DS. Prmt5, which forms distinct homo‐oligomers, is a member of the protein‐arginine methyltransferase family. J Biol Chem 2001; 276: 11393–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

