Fluxes in "free" and total zinc are essential for progression of intraerythrocytic stages of Plasmodium falciparum
- PMID: 22726687
- PMCID: PMC3601789
- DOI: 10.1016/j.chembiol.2012.04.013
Fluxes in "free" and total zinc are essential for progression of intraerythrocytic stages of Plasmodium falciparum
Abstract
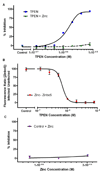
Dynamic fluxes in the concentration of ions and small molecules are fundamental features of cell signaling, differentiation, and development. Similar roles for fluxes in transition metal concentrations are less well established. Here, we show that massive zinc fluxes are essential in the infection cycle of an intracellular eukaryotic parasite. Using single-cell quantitative imaging, we show that growth of the blood-stage Plasmodium falciparum parasite requires acquisition of 30 million zinc atoms per erythrocyte before host cell rupture, corresponding to a 400% increase in total zinc concentration. Zinc accumulates in a freely available form in parasitophorous compartments outside the food vacuole, including mitochondria. Restriction of zinc availability via small molecule treatment causes a drop in mitochondrial membrane potential and severely inhibits parasite growth. Thus, extraordinary zinc acquisition and trafficking are essential for parasite development.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
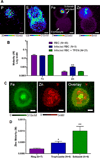
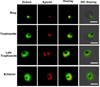
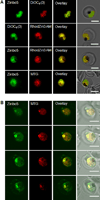
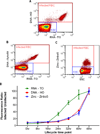

Comment in
-
Malarial parasites accumulate labile zinc pools.Chem Biol. 2012 Jun 22;19(6):660-1. doi: 10.1016/j.chembiol.2012.06.003. Chem Biol. 2012. PMID: 22726676
References
-
- Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol Today. 2000;16:427–433. - PubMed
-
- Berg JM. Zinc finger domains: hypotheses and current knowledge. Annu Rev Biophys Biophys Chem. 1990;19:405–421. - PubMed
-
- Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM038784/GM/NIGMS NIH HHS/United States
- HL079397/HL/NHLBI NIH HHS/United States
- HL69630/HL/NHLBI NIH HHS/United States
- P01 HL078826/HL/NHLBI NIH HHS/United States
- R01 AI039071/AI/NIAID NIH HHS/United States
- R01 HL079397/HL/NHLBI NIH HHS/United States
- R01 GM038784/GM/NIGMS NIH HHS/United States
- NCI CA060553/CA/NCI NIH HHS/United States
- GM038784/GM/NIGMS NIH HHS/United States
- HL078826/HL/NHLBI NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- R01 HL069630/HL/NHLBI NIH HHS/United States
- R01 GM038047/GM/NIGMS NIH HHS/United States
- GM38047/GM/NIGMS NIH HHS/United States
- AI39071/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

