Dynamic FoxG1 expression coordinates the integration of multipolar pyramidal neuron precursors into the cortical plate
- PMID: 22726835
- PMCID: PMC3653132
- DOI: 10.1016/j.neuron.2012.04.025
Dynamic FoxG1 expression coordinates the integration of multipolar pyramidal neuron precursors into the cortical plate
Abstract
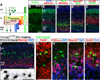
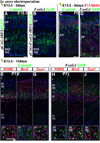
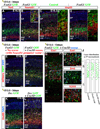
Pyramidal cells of the cerebral cortex are born in the ventricular zone and migrate through the intermediate zone to enter into the cortical plate. In the intermediate zone, these migrating precursors move tangentially and initiate the extension of their axons by transiently adopting a characteristic multipolar morphology. We observe that expression of the forkhead transcription factor FoxG1 is dynamically regulated during this transitional period. By utilizing conditional genetic strategies, we show that the downregulation of FoxG1 at the beginning of the multipolar cell phase induces Unc5D expression, the timing of which ultimately determines the laminar identity of pyramidal neurons. In addition, we demonstrate that the re-expression of FoxG1 is required for cells to transit out of the multipolar cell phase and to enter into the cortical plate. Thus, the dynamic expression of FoxG1 during migration within the intermediate zone is essential for the proper assembly of the cerebral cortex.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. - PubMed
-
- Alcantara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127:1359–1372. - PubMed
-
- Alfano C, Viola L, Heng JI, Pirozzi M, Clarkson M, Flore G, De Maio A, Schedl A, Guillemot F, Studer M. COUP-TFI promotes radial migration and proper morphology of callosal projection neurons by repressing Rnd2 expression. Development. 2011;138:4685–4697. - PubMed
-
- Angevine JBJ, Sidman RL. Autoradiographic Study of Cell Migration during Histogenesis of Cerebral Cortex in the Mouse. Nature. 1961;192:766–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

