Sensitive quantitative assays for tau and phospho-tau in transgenic mouse models
- PMID: 22727277
- PMCID: PMC3474864
- DOI: 10.1016/j.neurobiolaging.2012.05.010
Sensitive quantitative assays for tau and phospho-tau in transgenic mouse models
Abstract
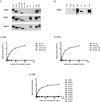
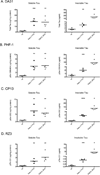
Transgenic mouse models have been an invaluable resource in elucidating the complex roles of β-amyloid and tau in Alzheimer's disease. Although many laboratories rely on qualitative or semiquantitative techniques when investigating tau pathology, we have developed 4 Low-Tau, Sandwich enzyme-linked immunosorbent assays (ELISAs) that quantitatively assess different epitopes of tau relevant to Alzheimer's disease: total tau, pSer-202, pThr-231, and pSer-396/404. In this study, after comparing our assays with commercially available ELISAs, we demonstrate our assay's high specificity and quantitative capabilities using brain homogenates from tau transgenic mice, htau, JNPL3, and tau knockout. All 4 ELISAs show excellent specificity for mouse and human tau, with no reactivity to tau knockout animals. An age-dependent increase of serum tau in both tau transgenic models was also seen. Taken together, these assays are valuable methods to quantify tau and phospho-tau levels in transgenic animals, by examining tau levels in brain and measuring tau as a potential serum biomarker.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86(3):582–590. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–639. - PubMed
-
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103(1):26–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

