The Akt-SRPK-SR axis constitutes a major pathway in transducing EGF signaling to regulate alternative splicing in the nucleus
- PMID: 22727668
- PMCID: PMC3418396
- DOI: 10.1016/j.molcel.2012.05.014
The Akt-SRPK-SR axis constitutes a major pathway in transducing EGF signaling to regulate alternative splicing in the nucleus
Erratum in
-
The Akt-SRPK-SR Axis Constitutes a Major Pathway in Transducing EGF Signaling to Regulate Alternative Splicing in the Nucleus.Mol Cell. 2018 Sep 6;71(5):872. doi: 10.1016/j.molcel.2018.08.024. Mol Cell. 2018. PMID: 30193100 Free PMC article. No abstract available.
Abstract
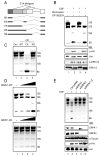
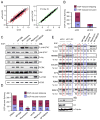
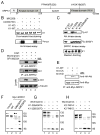
Pre-mRNA splicing is regulated by developmental and environmental cues, but little is known about how specific signals are transduced in mammalian cells to regulate this critical gene expression step. Here, we report massive reprogramming of alternative splicing in response to EGF signaling. By blocking individual branches in EGF signaling, we found that Akt activation plays a major role, while other branches, such as the JAK/STAT and ERK pathways, make minor contributions to EGF-induced splicing. Activated Akt next branches to SR protein-specific kinases, rather than mTOR, by inducing SRPK autophosphorylation that switches the splicing kinases from Hsp70- to Hsp90-containing complexes. This leads to enhanced SRPK nuclear translocation and SR protein phosphorylation. These findings reveal a major signal transduction pathway for regulated splicing and place SRPKs in a central position in the pathway, consistent with their reputed roles in a large number of human cancers.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Amin EM, Oltean S, Hua J, Gammons MV, Hamdollah-Zadeh M, Welsh GI, Cheung MK, Ni L, Kase S, Rennel ES, Symonds KE, Nowak DG, Royer-Pokora B, Saleem MA, Hagiwara M, Schumacher VA, Harper SJ, Hinton DR, Bates DO, Ladomery MR. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011;20:768–780. - PMC - PubMed
-
- Blaustein M, Pelisch F, Tanos T, Muñoz MJ, Wengier D, Quadrana L, Sanford JR, Muschietti JP, Kornblihtt AR, Cáceres JF, Coso OA, Srebrow A. Concerted regulation of nuclear and cytoplasmic activities of SR proteins by Akt. Nat Struct Mol Biol. 2005;12:1037–1044. - PubMed
-
- Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

