Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial
- PMID: 22727733
- PMCID: PMC12261288
- DOI: 10.1016/j.jhep.2012.06.014
Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial
Abstract
Background & aims: The Sorafenib Hepatocellular Carcinoma (HCC) Assessment Randomized Protocol (SHARP) trial demonstrated that sorafenib improves overall survival and is safe for patients with advanced HCC. In this trial, 602 patients with well-preserved liver function (>95% Child-Pugh A) were randomized to receive either sorafenib 400mg or matching placebo orally b.i.d. on a continuous basis. Because HCC is a heterogeneous disease, baseline patient characteristics may affect individual responses to treatment. In a comprehensive series of exploratory subgroup analyses, data from the SHARP trial were analyzed to discern if baseline patient characteristics influenced the efficacy and safety of sorafenib.
Methods: Five subgroup domains were assessed: disease etiology, tumor burden, performance status, tumor stage, and prior therapy. Overall survival (OS), time to progression (TTP), disease control rate (DCR), and safety were assessed for subgroups within each domain.
Results: Subgroup analyses showed that sorafenib consistently improved median OS compared with placebo, as reflected by hazard ratios (HRs) of 0.50-0.85, similar to the complete cohort (HR=0.69). Sorafenib also consistently improved median TTP (HR, 0.40-0.64), except in HBV-positive patients (HR, 1.03), and DCR. Results are limited by small patient numbers in some subsets. The most common grade 3/4 adverse events included diarrhea, hand-foot skin reaction, and fatigue; the incidence of which did not differ appreciably among subgroups.
Conclusions: These exploratory subgroup analyses showed that sorafenib consistently improved median OS and DCR compared with placebo in patients with advanced HCC, irrespective of disease etiology, baseline tumor burden, performance status, tumor stage, and prior therapy.
Copyright © 2012 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
Jordi Bruix has received honoraria and research funding from Bayer HealthCare Pharmaceuticals and consulting fees from Bayer HealthCare Pharmaceuticals, Onyx Pharmaceuticals, Biocompatibles, Bristol-Myers Squibb, Glaxo, Kowa, Novartis, and ArQule; Jean-Luc Raoul has received consulting fees from Bayer HealthCare Pharmaceuticals and Biocompatibles and lecture fees from Bayer HealthCare Pharmaceuticals; Vincenzo Mazzaferro has received consulting fees from Bayer HealthCare Pharmaceuticals; Luigi Bolondi has received consulting and lecture fees from Bayer HealthCare Pharmaceuticals; Peter R. Galle has received consulting and lecture fees from Bayer HealthCare Pharmaceuticals; Armando Santoro has received consulting fees from Bayer HealthCare Pharmaceuticals; Camillo Porta has received consulting and lecture fees and research Grants from Bayer HealthCare Pharmaceuticals; Jorge A. Marrero has received consulting fees from Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals; Andrea Nadel, Michael Shan, and Dimitris Voliotis are employees of Bayer HealthCare Pharmaceuticals; Marius Moscovici is an employee of Bayer Schering Pharma; Josep M. Llovet has received consulting fees from Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals and honoraria and research funding from Bayer HealthCare Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
Figures



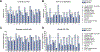
References
-
- Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. , Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698–711. - PubMed
-
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–1255. - PubMed
-
- Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200–207. - PubMed
-
- Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008;48:S20–S37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

