The effect on quadruplex stability of North-nucleoside derivatives in the loops of the thrombin-binding aptamer
- PMID: 22727781
- PMCID: PMC3534854
- DOI: 10.1016/j.bmc.2012.06.005
The effect on quadruplex stability of North-nucleoside derivatives in the loops of the thrombin-binding aptamer
Abstract
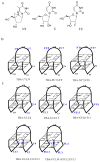
Modified thrombin-binding aptamers (TBAs) carrying uridine (U), 2'-deoxy-2'-fluorouridine (FU) and North-methanocarbathymidine (NT) residues in the loop regions were synthesized and analyzed by UV thermal denaturation experiments and CD spectroscopy. The replacement of thymidines in the TGT loop by U and FU results in an increased stability of the antiparallel quadruplex structure described for the TBA while the presence of NT residues in the same positions destabilizes the antiparallel structure. The substitution of the thymidines in the TT loops for U, FU and NT induce a destabilization of the antiparallel quadruplex, indicating the crucial role of these positions. NMR studies on TBAs modified with uridines at the TGT loop also confirm the presence of the antiparallel quadruplex structure. Nevertheless, replacement of two Ts in the TT loops by uridine gives a more complex scenario in which the antiparallel quadruplex structure is present along with other partially unfolded species or aggregates.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

