Severe spruelike enteropathy associated with olmesartan
- PMID: 22728033
- PMCID: PMC3538487
- DOI: 10.1016/j.mayocp.2012.06.003
Severe spruelike enteropathy associated with olmesartan
Abstract
Objective: To report the response to discontinuation of olmesartan, an angiotensin II receptor antagonist commonly prescribed for treatment of hypertension, in patients with unexplained severe spruelike enteropathy.
Patients and methods: All 22 patients included in this report were seen at Mayo Clinic in Rochester, Minnesota, between August 1, 2008, and August 1, 2011, for evaluation of unexplained chronic diarrhea and enteropathy while taking olmesartan. Celiac disease was ruled out in all cases. To be included in the study, the patients also had to have clinical improvement after suspension of olmesartan.
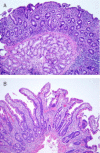
Results: The 22 patients (13 women) had a median age of 69.5 years (range, 47-81 years). Most patients were taking 40 mg/d of olmesartan (range, 10-40 mg/d). The clinical presentation was of chronic diarrhea and weight loss (median, 18 kg; range, 2.5-57 kg), which required hospitalization in 14 patients (64%). Intestinal biopsies showed both villous atrophy and variable degrees of mucosal inflammation in 15 patients, and marked subepithelial collagen deposition (collagenous sprue) in 7. Tissue transglutaminase antibodies were not detected. A gluten-free diet was not helpful. Collagenous or lymphocytic gastritis was documented in 7 patients, and microscopic colitis was documented in 5 patients. Clinical response, with a mean weight gain of 12.2 kg, was demonstrated in all cases. Histologic recovery or improvement of the duodenum after discontinuation of olmesartan was confirmed in all 18 patients who underwent follow-up biopsies.
Conclusion: Olmesartan may be associated with a severe form of spruelike enteropathy. Clinical response and histologic recovery are expected after suspension of the drug.
Copyright © 2012 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Olmesartan and intestinal adverse effects in the ROADMAP study.Mayo Clin Proc. 2012 Dec;87(12):1230-1; author reply 1232. doi: 10.1016/j.mayocp.2012.10.005. Mayo Clin Proc. 2012. PMID: 23218089 Free PMC article. No abstract available.
-
Small bowel histopathologic findings suggestive of celiac disease in an asymptomatic patient receiving olmesartan.Mayo Clin Proc. 2012 Dec;87(12):1231-2; author reply 1232. doi: 10.1016/j.mayocp.2012.09.011. Mayo Clin Proc. 2012. PMID: 23218090 Free PMC article. No abstract available.
References
-
- The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . National Institutes of Health, National Heart, Lung, and Blood Institute, US Department of Health and Human Services; Bethesda, MD: 2004. NIH Publication No. 04-5230. - PubMed
-
- Ziegler T.R., Fernández-Estívariz C., Gu L.H., Fried M.W., Leader L.M. Severe villus atrophy and chronic malabsorption induced by azathioprine. Gastroenterology. 2003;124(7):1950–1957. - PubMed
-
- Kamar N., Faure P., Dupuis E. Villous atrophy induced by mycophenolate mofetil in renal-transplant patients. Transpl Int. 2004;17(8):463–467. - PubMed
-
- Weclawiak H., Ould-Mohamed A., Bournet B. Duodenal villous atrophy: a cause of chronic diarrhea after solid-organ transplantation. Am J Transplant. 2011;11(3):575–582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

