NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing
- PMID: 22728064
- PMCID: PMC3409433
- DOI: 10.1016/j.ajpath.2012.04.010
NK cells modulate the inflammatory response to corneal epithelial abrasion and thereby support wound healing
Abstract
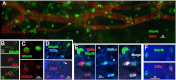
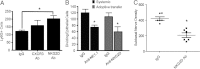
Natural killer (NK) cells are lymphocytes of the innate immune system that have crucial cytotoxic and regulatory roles in adaptive immunity and inflammation. Herein, we consider a role for these cells in corneal wound healing. After a 2-mm central epithelial abrasion of the mouse cornea, a subset of classic NK cells migrated into the limbus and corneal stroma, peaking at 24 hours with an eightfold increase over baseline. Depletion of γδ T cells significantly reduced NK cell accumulation (>70%; P < 0.01); however, in neutrophil-depleted animals, NK cell influx was normal. Isolated spleen NK cells migrated to the wounded cornea, and this migration was reduced by greater than 60% (P < 0.01) by ex vivo antibody blocking of NK cell CXCR3 or CCR2. Antibody-induced depletion of NK cells significantly altered the inflammatory reaction to corneal wounding, as evidenced by a 114% increase (P < 0.01) in neutrophil influx at a time when acute inflammation is normally waning. Functional blocking of NKG2D, an activating receptor for NK cell cytotoxicity and cytokine secretion, did not inhibit NK cell immigration, but significantly increased neutrophil influx. Consistent with excessive neutrophil accumulation, NK depletion and blocking of NKG2D also inhibited corneal nerve regeneration and epithelial healing (P < 0.01). Findings of this study suggest that NK cells are actively involved in corneal healing by limiting the innate acute inflammatory reaction to corneal wounding.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Yamagami S., Hamrah P., Miyamoto K., Miyazaki D., Dekaris I., Dawson T., Lu B., Gerard C., Dana M.R. CCR5 chemokine receptor mediates recruitment of MHC class II-positive Langerhans cells in the mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2005;46:1201–1207. - PubMed
-
- Hamrah P., Huq S.O., Liu Y., Zhang Q., Dana M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

