Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice
- PMID: 22728083
- PMCID: PMC3597773
- DOI: 10.1016/j.jaci.2012.05.009
Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice
Abstract
Background: Mast cells express receptors for complement anaphylatoxins C3a and C5a (ie, C3a receptor [C3aR] and C5a receptor [C5aR]), and C3a and C5a are generated during various IgE-dependent immediate hypersensitivity reactions in vivo. However, it is not clear to what extent mast cell expression of C3aR or C5aR influences C3a- or C5a-induced cutaneous responses or IgE-dependent mast cell activation and passive cutaneous anaphylaxis (PCA) in vivo.
Objective: We sought to assess whether mouse skin mast cell expression of C3aR or C5aR influences (1) the cells' responsiveness to intradermal injections of C3a or C5a or (2) the extent of IgE-dependent mast cell degranulation and PCA in vivo.
Methods: We measured the magnitude of cutaneous responses to intradermal injections of C3a or C5a and the extent of IgE-dependent mast cell degranulation and PCA responses in mice containing mast cells that did or did not express C3aR or C5aR.
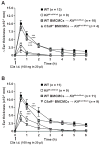
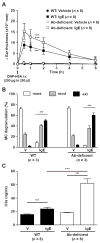
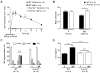
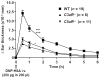
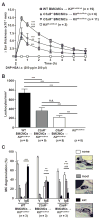
Results: The majority of the skin swelling induced by means of intradermal injection of C3a or C5a required that mast cells at the site expressed C3aR or C5aR, respectively, and the extent of IgE-dependent degranulation of skin mast cells and IgE-dependent PCA was significantly reduced when mast cells lacked either C3aR or C5aR. IgE-dependent PCA responses associated with local increases in C3a levels occurred in antibody-deficient mice but not in mice deficient in FcɛRIγ.
Conclusion: Expression of C3aR and C5aR by skin mast cells contributes importantly to the ability of C3a and C5a to induce skin swelling and can enhance mast cell degranulation and inflammation during IgE-dependent PCA in vivo.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Conflict of interest statement
The authors state no conflict of interest.
Figures
References
-
- Simons FE, Frew AJ, Ansotegui IJ, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120:S2–24. - PubMed
-
- Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: an investigation into its epidemiology. Arch Intern Med. 2001;161:15–21. - PubMed
-
- Kalesnikoff J, Galli SJ. Anaphylaxis: mechanisms of mast cell activation. Chem Immunol Allergy. 2010;95:45–66. - PubMed
-
- Friedberger E. Kritik der Theorien ueber die Anaphylaxie. Zeitschrift fuer Immunitaetsforschung und experimentelle Therapie, II. 1909:208–24.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

