Increased NADPH oxidase-derived superoxide is involved in the neuronal cell death induced by hypoxia-ischemia in neonatal hippocampal slice cultures
- PMID: 22728269
- PMCID: PMC3527086
- DOI: 10.1016/j.freeradbiomed.2012.06.012
Increased NADPH oxidase-derived superoxide is involved in the neuronal cell death induced by hypoxia-ischemia in neonatal hippocampal slice cultures
Abstract
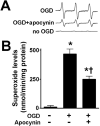
Neonatal brain hypoxia-ischemia (HI) results in neuronal cell death. Previous studies indicate that reactive oxygen species, such as superoxide, play a key role in this process. However, the cellular sources have not been established. In this study we examine the role of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex in neonatal HI brain injury and elucidate its mechanism of activation. Rat hippocampal slices were exposed to oxygen glucose deprivation (OGD) to mimic the conditions seen in HI. Initial studies confirmed an important role for NADPH oxidase-derived superoxide in the oxidative stress associated with OGD. Further, the OGD-mediated increase in apoptotic cell death was inhibited by the NADPH oxidase inhibitor apocynin. The activation of NADPH oxidase was found to be dependent on the p38 mitogen-activated protein kinase-mediated phosphorylation and activation of the p47(phox) subunit. Using an adeno-associated virus antisense construct to selectively decrease p47(phox) expression in neurons showed that this led to inhibition of both the increase in superoxide and the neuronal cell death associated with OGD. We also found that NADPH oxidase inhibition in a neonatal rat model of HI or scavenging hydrogen peroxide reduced brain injury. Thus, we conclude that activation of the NADPH oxidase complex contributes to the oxidative stress during HI and that therapies targeted against this complex could provide neuroprotection against the brain injury associated with neonatal HI.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures














References
-
- Glass HC, Ferriero DM. Treatment of hypoxic-ischemic encephalopathy in newborns. Curr Treat Options Neurol. 2007;9:414–423. - PubMed
-
- Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28:1353–1365. - PubMed
-
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. - PubMed
-
- Martin LJ, Brambrink AM, Price AC, Kaiser A, Agnew DM, Ichord RN, Traystman RJ. Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and evolves with oxidative stress. Neurobiol Dis. 2000;7:169–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

