Switchable molecular magnets
- PMID: 22728438
- PMCID: PMC3410140
- DOI: 10.2183/pjab.88.213
Switchable molecular magnets
Abstract
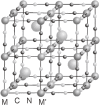
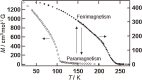
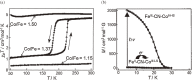
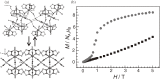
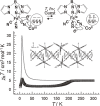
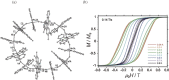
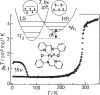
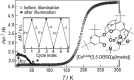
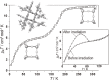
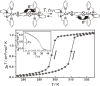
Various molecular magnetic compounds whose magnetic properties can be controlled by external stimuli have been developed, including electrochemically, photochemically, and chemically tunable bulk magnets as well as a phototunable antiferromagnetic phase of single chain magnet. In addition, we present tunable paramagnetic mononuclear complexes ranging from spin crossover complexes and valence tautomeric complexes to Co complexes in which orbital angular momentum can be switched. Furthermore, we recently developed several switchable clusters and one-dimensional coordination polymers. The switching of magnetic properties can be achieved by modulating metals, ligands, and molecules/ions in the second sphere of the complexes.
Figures


References
-
- Iwamura H. (2005) Organic-synthetic and supramolecular approaches to free radical-based magnets. Proc. Jpn. Acad., Ser. B 81, 233–243
-
- Itoh K., Takui T. (2004) High spin chemistry underlying organic molecular magnetism. Topological symmetry rule as the first principle of spin alignment in organic open-shell systems of π-conjugation and their ions. Proc. Jpn. Acad., Ser. B 80, 29–40
-
- Kinoshita M. (2004) π-Electron ferromagnetism of a purely organic radical crystal. Proc. Jpn. Acad., Ser. B 80, 41–53
-
- Kahn, O. (1993) Molecular Magnetism. VCH, New York.
-
- Verdaguer M., Bleuzen A., Marvaud V., Vaissermann J., Seuleiman M., Desplanches C., Scuiller A., Train C., Garde R., Gelly G., Lomenech C., Rosenman I., Veillet P., Cartier C., Villain F. (1999) Molecules to build solids: high Tc molecule-based magnets by design and recent revival of cyano complexes chemistry. Coord. Chem. Rev. 192, 1023–1047
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

