Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains
- PMID: 22728824
- PMCID: PMC3411076
- DOI: 10.1038/emboj.2012.171
Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains
Abstract
K(2P)2.1 (TREK-1) is a polymodal two-pore domain leak potassium channel that responds to external pH, GPCR-mediated phosphorylation signals, and temperature through the action of distinct sensors within the channel. How the various intracellular and extracellular sensory elements control channel function remains unresolved. Here, we show that the K(2P)2.1 (TREK-1) intracellular C-terminal tail (Ct), a major sensory element of the channel, perceives metabolic and thermal commands and relays them to the extracellular C-type gate through transmembrane helix M4 and pore helix 1. By decoupling Ct from the pore-forming core, we further demonstrate that Ct is the primary heat-sensing element of the channel, whereas, in contrast, the pore domain lacks robust temperature sensitivity. Together, our findings outline a mechanism for signal transduction within K(2P)2.1 (TREK-1) in which there is a clear crosstalk between the C-type gate and intracellular Ct domain. In addition, our findings support the general notion of the existence of modular temperature-sensing domains in temperature-sensitive ion channels. This marked distinction between gating and sensory elements suggests a general design principle that may underlie the function of a variety of temperature-sensitive channels.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

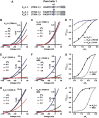


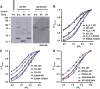

References
-
- Arias JM, Murbartian J, Vitko I, Lee JH, Perez-Reyes E (2005) Transfer of beta subunit regulation from high to low voltage-gated Ca2+ channels. FEBS Lett 579: 3907–3912 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

