Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells
- PMID: 22729031
- PMCID: PMC3467331
- DOI: 10.1038/nbt.2247
Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells
Abstract
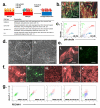
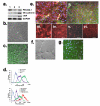
The blood-brain barrier (BBB) is crucial to the health of the brain and is often compromised in neurological disease. Moreover, because of its barrier properties, this endothelial interface restricts uptake of neurotherapeutics. Thus, a renewable source of human BBB endothelium could spur brain research and pharmaceutical development. Here we show that endothelial cells derived from human pluripotent stem cells (hPSCs) acquire BBB properties when co-differentiated with neural cells that provide relevant cues, including those involved in Wnt/β-catenin signaling. The resulting endothelial cells have many BBB attributes, including well-organized tight junctions, appropriate expression of nutrient transporters and polarized efflux transporter activity. Notably, they respond to astrocytes, acquiring substantial barrier properties as measured by transendothelial electrical resistance (1,450 ± 140 Ω cm2), and they possess molecular permeability that correlates well with in vivo rodent blood-brain transfer coefficients.
Figures


References
-
- Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Molecular interventions. 2003;3:90–105. 151. - PubMed
-
- Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochimica et biophysica acta. 2009;1788:842–857. - PubMed
-
- Syvanen S, et al. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:635–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

