EMMPRIN is secreted by human uterine epithelial cells in microvesicles and stimulates metalloproteinase production by human uterine fibroblast cells
- PMID: 22729071
- PMCID: PMC4046446
- DOI: 10.1177/1933719112450332
EMMPRIN is secreted by human uterine epithelial cells in microvesicles and stimulates metalloproteinase production by human uterine fibroblast cells
Erratum in
-
Corrigendum.Reprod Sci. 2015 Jan;22(1):132. doi: 10.1177/1933719113508910. Epub 2013 Oct 15. Reprod Sci. 2015. PMID: 24129038 Free PMC article. No abstract available.
Abstract
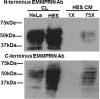
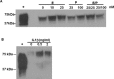
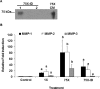
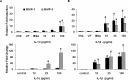
Endometrial remodeling is a physiological process involved in the gynecological disease, endometriosis. Tissue remodeling is directed by uterine fibroblast production of matrix metalloproteinases (MMPs). Several MMPs are regulated directly by the protein extracellular matrix metalloproteinase inducer (EMMPRIN) and also by proinflammatory cytokines such as interleukin (IL)1-α/β. We hypothesized that human uterine epithelial cells (HESs) secrete intact EMMPRIN to stimulate MMPs. Microvesicles from HES cell-conditioned medium (CM) expressed intact EMMPRIN protein. Treatment of HES cells with estradiol or phorbyl 12-myristate-13-acetate increased the release of EMMPRIN-containing microvesicles. The HES CM stimulated MMP-1, -2, and -3 messenger RNA levels in human uterine fibroblasts (HUFs) and EMMPRIN immunodepletion from HES-cell concentrated CM reduced MMP stimulation (P < .05). Treatment of HUF cells with low concentrations of IL-1β/α stimulated MMP production (P < .05). These results indicate that HES cells regulate MMP production by HUF cells by secretion of EMMPRIN, in response to ovarian hormones, proinflammatory cytokines as well as activation of protein kinase C.
Conflict of interest statement
Figures


Similar articles
-
Extracellular matrix metalloproteinase inducer regulates metalloproteinases in human uterine endometrium.J Clin Endocrinol Metab. 2006 Jun;91(6):2358-65. doi: 10.1210/jc.2005-0601. Epub 2006 Mar 7. J Clin Endocrinol Metab. 2006. PMID: 16522689
-
Cytokines regulate matrix metalloproteinases in human uterine endometrial fibroblast cells through a mechanism that does not involve increases in extracellular matrix metalloproteinase inducer.Am J Reprod Immunol. 2006 Sep;56(3):201-14. doi: 10.1111/j.1600-0897.2006.00418.x. Am J Reprod Immunol. 2006. PMID: 16911716
-
Stimulation of GPR30 increases release of EMMPRIN-containing microvesicles in human uterine epithelial cells.J Clin Endocrinol Metab. 2012 Dec;97(12):4613-22. doi: 10.1210/jc.2012-2098. Epub 2012 Sep 25. J Clin Endocrinol Metab. 2012. PMID: 23012390 Free PMC article.
-
Extracellular matrix metalloproteinase inducer in brain ischemia and intracerebral hemorrhage.Front Immunol. 2022 Aug 31;13:986469. doi: 10.3389/fimmu.2022.986469. eCollection 2022. Front Immunol. 2022. PMID: 36119117 Free PMC article. Review.
-
The role of basigin in reproduction.Reproduction. 2020 Feb;159(2):R97-R109. doi: 10.1530/REP-19-0268. Reproduction. 2020. PMID: 31600731 Review.
Cited by
-
The Role of Extracellular Vesicles in Embryo Implantation.Front Endocrinol (Lausanne). 2022 Jan 28;13:809596. doi: 10.3389/fendo.2022.809596. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35154016 Free PMC article. Review.
-
Autotaxin-mediated lipid signaling intersects with LIF and BMP signaling to promote the naive pluripotency transcription factor program.Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12478-12483. doi: 10.1073/pnas.1608564113. Epub 2016 Oct 13. Proc Natl Acad Sci U S A. 2016. PMID: 27738243 Free PMC article.
-
Extracellular Vesicles in Human Oogenesis and Implantation.Int J Mol Sci. 2019 May 1;20(9):2162. doi: 10.3390/ijms20092162. Int J Mol Sci. 2019. PMID: 31052401 Free PMC article. Review.
-
Loss of basigin expression in uterine cells leads to subfertility in female mice†.Biol Reprod. 2021 Oct 11;105(4):859-875. doi: 10.1093/biolre/ioab109. Biol Reprod. 2021. PMID: 34106247 Free PMC article.
-
Biologia futura: embryo-maternal communication via progesterone-induced blocking factor (PIBF) positive embryo-derived extracellular vesicles. Their role in maternal immunomodulation.Biol Futur. 2021 Mar;72(1):69-74. doi: 10.1007/s42977-020-00060-2. Epub 2021 Jan 29. Biol Futur. 2021. PMID: 34554496 Review.
References
-
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428–465 - PubMed
-
- Chou CS, Tai CJ, MacCalman CD, Leung PC. Dose-dependent effects of gonadotropin releasing hormone on matrix metalloproteinase (MMP)-2, and MMP-9 and tissue specific inhibitor of metalloproteinases-1 messenger ribonucleic acid levels in human decidual stromal cells in vitro. J Clin Endocrinol Metab. 2003;88(2):680–688 - PubMed
-
- Dong JC, Dong H, Campana A, Bischof P. Matrix metalloproteinases and their specific tissue inhibitors in menstruation. Reproduction. 2002;123(5):621–631 - PubMed
-
- Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87(10):4782–4791 - PubMed
-
- Chen L, Nakai M, Belton RJ, Jr, Nowak RA. Expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinases during mouse embryonic development. Reproduction. 2007;133(2):405–414 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

