ER network formation requires a balance of the dynamin-like GTPase Sey1p and the Lunapark family member Lnp1p
- PMID: 22729086
- PMCID: PMC3389217
- DOI: 10.1038/ncb2523
ER network formation requires a balance of the dynamin-like GTPase Sey1p and the Lunapark family member Lnp1p
Abstract
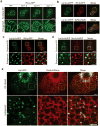
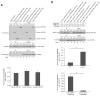
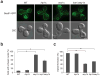
Although studies on endoplasmic reticulum (ER) structure and dynamics have focused on the ER tubule-forming proteins (reticulons and DP1/Yop1p) and the tubule fusion protein atlastin, nothing is known about the proteins and processes that act to counterbalance this machinery. Here we show that Lnp1p, a member of the conserved Lunapark family, plays a role in ER network formation. Lnp1p binds to the reticulons and Yop1p and resides at ER tubule junctions in both yeast and mammalian cells. In the yeast Saccharomyces cerevisiae, the interaction of Lnp1p with the reticulon protein, Rtn1p, and the localization of Lnp1p to ER junctions are regulated by Sey1p, the yeast orthologue of atlastin. We propose that Lnp1p and Sey1p act antagonistically to balance polygonal network formation. In support of this proposal, we show that the collapsed, densely reticulated ER network in lnp1 Δ cells is partially restored when the GTPase activity of Sey1p is abrogated.
Figures





References
-
- Du Y, Ferro-Novick S, Novick P. Dynamics and inheritance of the endoplasmic reticulum. J Cell Sci. 2004;117:2871–2878. - PubMed
-
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. - PubMed
-
- Lee C, Chen LB. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988;54:37–46. - PubMed
-
- Hu J, et al. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science. 2008;319:1247–1250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

