Metallo-β-lactamases withstand low Zn(II) conditions by tuning metal-ligand interactions
- PMID: 22729148
- PMCID: PMC3470787
- DOI: 10.1038/nchembio.1005
Metallo-β-lactamases withstand low Zn(II) conditions by tuning metal-ligand interactions
Abstract
A number of multiresistant bacterial pathogens inactivate antibiotics by producing Zn(II)-dependent β-lactamases. We show that metal uptake leading to an active dinuclear enzyme in the periplasmic space of Gram-negative bacteria is ensured by a cysteine residue, an unusual metal ligand in oxidizing environments. Kinetic, structural and affinity data show that such Zn(II)-cysteine interaction is an adaptive trait that tunes the metal binding affinity, thus enabling antibiotic resistance at restrictive Zn(II) concentrations.
Figures

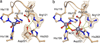

References
-
- Crowder MW, Spencer J, Vila AJ. Metallo-beta-lactamases: Novel Weaponry for Antibiotic Resistance in Bacteria. Acc. Chem. Res. 2006;39:721–728. - PubMed
-
- Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to beta-lactam antibiotics: compelling opportunism, compelling opportunity. Chem Rev. 2005;105:395–424. - PubMed
-
- Fabiane SM, et al. Crystal structure of the zinc-dependent beta lactamase from Bacillus cereus at 1.9 A resolution: binuclear active site with features of a mononuclear enzyme. Biochemistry. 1998;37:12404–12411. - PubMed
-
- Orellano EG, Girardini JE, Cricco JA, Ceccarelli EA, Vila AJ. Spectroscopic characterization of a binuclear metal site in Bacillus cereus beta-lactamase II. Biochemistry. 1998;37:10173–10180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

