The periplasmic nitrate reductase nap is required for anaerobic growth and involved in redox control of magnetite biomineralization in Magnetospirillum gryphiswaldense
- PMID: 22730130
- PMCID: PMC3430331
- DOI: 10.1128/JB.00903-12
The periplasmic nitrate reductase nap is required for anaerobic growth and involved in redox control of magnetite biomineralization in Magnetospirillum gryphiswaldense
Abstract
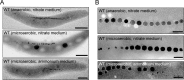
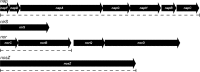
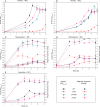
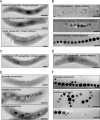
The magnetosomes of many magnetotactic bacteria consist of membrane-enveloped magnetite crystals, whose synthesis is favored by a low redox potential. However, the cellular redox processes governing the biomineralization of the mixed-valence iron oxide have remained unknown. Here, we show that in the alphaproteobacterium Magnetospirillum gryphiswaldense, magnetite biomineralization is linked to dissimilatory nitrate reduction. A complete denitrification pathway, including gene functions for nitrate (nap), nitrite (nir), nitric oxide (nor), and nitrous oxide reduction (nos), was identified. Transcriptional gusA fusions as reporters revealed that except for nap, the highest expression of the denitrification genes coincided with conditions permitting maximum magnetite synthesis. Whereas microaerobic denitrification overlapped with oxygen respiration, nitrate was the only electron acceptor supporting growth in the entire absence of oxygen, and only the deletion of nap genes, encoding a periplasmic nitrate reductase, and not deletion of nor or nos genes, abolished anaerobic growth and also delayed aerobic growth in both nitrate and ammonium media. While loss of nosZ or norCB had no or relatively weak effects on magnetosome synthesis, deletion of nap severely impaired magnetite biomineralization and resulted in fewer, smaller, and irregular crystals during denitrification and also microaerobic respiration, probably by disturbing the proper redox balance required for magnetite synthesis. In contrast to the case for the wild type, biomineralization in Δnap cells was independent of the oxidation state of carbon substrates. Altogether, our data demonstrate that in addition to its essential role in anaerobic respiration, the periplasmic nitrate reductase Nap has a further key function by participating in redox reactions required for magnetite biomineralization.
Figures
References
-
- Bazylinski DA, Frankel R, Jannasch HW. 1988. Anaerobic magnetite production by a marine magnetotactic bacterium. Nature 334:518–519
-
- Bazylinski DA, Williams T. 2006. Ecophysiology of magnetotactic bacteria, p 64 In Schüler D. (ed), Magnetoreception and magnetosomes in bacteria. Springer Verlag, Heidelberg, Germany
-
- Bedmar EJ, Robles EF, Delgado MJ. 2005. The complete denitrification pathway of the symbiotic, nitrogen-fixing bacterium Bradyrhizobium japonicum. Biochem. Soc. Trans. 33:141–144 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

