Interactions between the NO-citrulline cycle and brain-derived neurotrophic factor in differentiation of neural stem cells
- PMID: 22730318
- PMCID: PMC3436173
- DOI: 10.1074/jbc.M111.338095
Interactions between the NO-citrulline cycle and brain-derived neurotrophic factor in differentiation of neural stem cells
Abstract
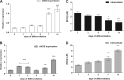
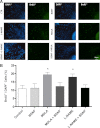
The diffusible messenger NO plays multiple roles in neuroprotection, neurodegeneration, and brain plasticity. Argininosuccinate synthase (AS) is a ubiquitous enzyme in mammals and the key enzyme of the NO-citrulline cycle, because it provides the substrate L-arginine for subsequent NO synthesis by inducible, endothelial, and neuronal NO synthase (NOS). Here, we provide evidence for the participation of AS and of the NO-citrulline cycle in the progress of differentiation of neural stem cells (NSC) into neurons, astrocytes, and oligodendrocytes. AS expression and activity and neuronal NOS expression, as well as l-arginine and NO(x) production, increased along neural differentiation, whereas endothelial NOS expression was augmented in conditions of chronic NOS inhibition during differentiation, indicating that this NOS isoform is amenable to modulation by extracellular cues. AS and NOS inhibition caused a delay in the progress of neural differentiation, as suggested by the decreased percentage of terminally differentiated cells. On the other hand, BDNF reversed the delay of neural differentiation of NSC caused by inhibition of NO(x) production. A likely cause is the lack of NO, which up-regulated p75 neurotrophin receptor expression, a receptor required for BDNF-induced differentiation of NSC. We conclude that the NO-citrulline cycle acts together with BDNF for maintaining the progress of neural differentiation.
Figures








References
-
- Moroz L. L., Gillette R., Sweedler J. V. (1999) Single-cell analyses of nitrergic neurons in simple nervous systems. J. Exp. Biol. 202, 333–341 - PubMed
-
- Reynolds B. A., Weiss S. (1996) Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 175, 1–13 - PubMed
-
- McKay R. (1997) Stem cells in the central nervous system. Science 276, 66–71 - PubMed
-
- Gage F. H. (2000) Mammalian neural stem cells. Science 287, 1433–1438 - PubMed
-
- Trujillo C. A., Schwindt T. T., Martins A. H., Alves J. M., Mello L. E., Ulrich H. (2009) Novel perspectives of neural stem cell differentiation. From neurotransmitters to therapeutics. Cytometry A 75, 38–53 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

