Autoregulation of Musashi1 mRNA translation during Xenopus oocyte maturation
- PMID: 22730340
- PMCID: PMC3845664
- DOI: 10.1002/mrd.22060
Autoregulation of Musashi1 mRNA translation during Xenopus oocyte maturation
Abstract
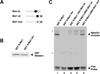
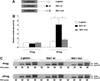
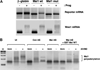
The mRNA translational control protein, Musashi, plays a critical role in cell fate determination through sequence-specific interactions with select target mRNAs. In proliferating stem cells, Musashi exerts repression of target mRNAs to promote cell cycle progression. During stem cell differentiation, Musashi target mRNAs are de-repressed and translated. Recently, we have reported an obligatory requirement for Musashi to direct translational activation of target mRNAs during Xenopus oocyte meiotic cell cycle progression. Despite the importance of Musashi in cell cycle regulation, only a few target mRNAs have been fully characterized. In this study, we report the identification and characterization of a new Musashi target mRNA in Xenopus oocytes. We demonstrate that progesterone-stimulated translational activation of the Xenopus Musashi1 mRNA is regulated through a functional Musashi binding element (MBE) in the Musashi1 mRNA 3' untranslated region (3' UTR). Mutational disruption of the MBE prevented translational activation of Musashi1 mRNA and its interaction with Musashi protein. Further, elimination of Musashi function through microinjection of inhibitory antisense oligonucleotides prevented progesterone-induced polyadenylation and translation of the endogenous Musashi1 mRNA. Thus, Xenopus Musashi proteins regulate translation of the Musashi1 mRNA during oocyte maturation. Our results indicate that the hierarchy of sequential and dependent mRNA translational control programs involved in directing progression through meiosis are reinforced by an intricate series of nested, positive feedback loops, including Musashi mRNA translational autoregulation. These autoregulatory positive feedback loops serve to amplify a weak initiating signal into a robust commitment for the oocyte to progress through the cell cycle and become competent for fertilization.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- Barnard DC, Ryan K, Manley JL, Richter JD. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell. 2004;119:641–651. - PubMed
-
- Battelli C, Nikopoulos GN, Mitchell JG, Verdi JM. The RNA-binding protein Musashi-1 regulates neural development through the translational repression of p21(WAF-1) Mol Cell Neurosci. 2006;31:85–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

