Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy
- PMID: 22730442
- PMCID: PMC3434870
- DOI: 10.1161/CIRCRESAHA.112.268128
Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy
Abstract
Rationale: Decreased fatty acid oxidation (FAO) with increased reliance on glucose are hallmarks of metabolic remodeling that occurs in pathological cardiac hypertrophy and is associated with decreased myocardial energetics and impaired cardiac function. To date, it has not been tested whether prevention of the metabolic switch that occurs during the development of cardiac hypertrophy has unequivocal benefits on cardiac function and energetics.
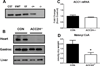
Objective: Because malonyl CoA production via acetyl CoA carboxylase 2 (ACC2) inhibits the entry of long chain fatty acids into the mitochondria, we hypothesized that mice with a cardiac-specific deletion of ACC2 (ACC2H-/-) would maintain cardiac FAO and improve function and energetics during the development of pressure-overload hypertrophy.
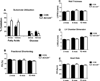
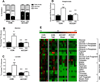
Methods and results: ACC2 deletion led to a significant reduction in cardiac malonyl CoA levels. In isolated perfused heart experiments, left ventricular function and oxygen consumption were similar in ACC2H-/- mice despite an ≈60% increase in FAO compared with controls (CON). After 8 weeks of pressure overload via transverse aortic constriction (TAC), ACC2H-/- mice exhibited a substrate utilization profile similar to sham animals, whereas CON-TAC hearts had decreased FAO with increased glycolysis and anaplerosis. Myocardial energetics, assessed by 31P nuclear magnetic resonance spectroscopy, and cardiac function were maintained in ACC2H-/- after 8 weeks of TAC. Furthermore, ACC2H-/--TAC demonstrated an attenuation of cardiac hypertrophy with a significant reduction in fibrosis relative to CON-TAC.
Conclusions: These data suggest that reversion to the fetal metabolic profile in chronic pathological hypertrophy is associated with impaired myocardial function and energetics and maintenance of the inherent cardiac metabolic profile and mitochondrial oxidative capacity is a viable therapeutic strategy.
Figures




Comment in
-
Metabolic remodeling in the hypertrophic heart: fuel for thought.Circ Res. 2012 Aug 31;111(6):666-8. doi: 10.1161/CIRCRESAHA.112.277392. Circ Res. 2012. PMID: 22935530 Free PMC article. No abstract available.
References
-
- Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. - PubMed
-
- Akki A, Smith K, Seymour AM. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem. 2008;311:215–224. - PubMed
-
- Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. - PubMed
-
- Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O'Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009;104:805–812. - PMC - PubMed
-
- Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

