3-Helix micelles stabilized by polymer springs
- PMID: 22731391
- PMCID: PMC4186246
- DOI: 10.1021/ja3048128
3-Helix micelles stabilized by polymer springs
Abstract
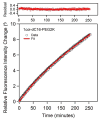
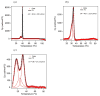
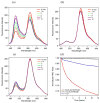
Despite increasing demands to employ amphiphilic micelles as nanocarriers and nanoreactors, it remains a significant challenge to simultaneously reduce the particle size and enhance the particle stability. Complementary to covalent chemical bonding and attractive intermolecular interactions, entropic repulsion can be incorporated by rational design in the headgroup of an amphiphile to generate small micelles with enhanced stability. A new family of amphiphilic peptide-polymer conjugates is presented where the hydrophilic headgroup is composed of a 3-helix coiled coil with poly(ethylene glycol) attached to the exterior of the helix bundle. When micelles form, the PEG chains are confined in close proximity and are compressed to act as a spring to generate lateral pressure. The formation of 3-helix bundles determines the location and the directionalities of the force vector of each PEG elastic spring so as to slow down amphiphile desorption. Since each component of the amphiphile can be readily tailored, these micelles provide numerous opportunities to meet current demands for organic nanocarriers with tunable stability in life science and energy science. Furthermore, present studies open new avenues to use energy arising from entropic polymer chain deformation to self-assemble energetically stable, single nanoscopic objects, much like repulsion that stabilizes bulk assemblies of colloidal particles.
Figures





Similar articles
-
Long-circulating 15 nm micelles based on amphiphilic 3-helix peptide-PEG conjugates.ACS Nano. 2012 Jun 26;6(6):5320-9. doi: 10.1021/nn301142r. Epub 2012 May 4. ACS Nano. 2012. PMID: 22545944 Free PMC article.
-
New design of helix bundle peptide-polymer conjugates.Biomacromolecules. 2008 Aug;9(8):2111-7. doi: 10.1021/bm800113g. Epub 2008 Jul 16. Biomacromolecules. 2008. PMID: 18627200 Free PMC article.
-
Micelle stabilization via entropic repulsion: balance of force directionality and geometric packing of subunit.Biomacromolecules. 2015 Mar 9;16(3):743-7. doi: 10.1021/bm501659w. Epub 2015 Jan 30. Biomacromolecules. 2015. PMID: 25575164
-
Poly(ethylene glycol)-polypeptide Copolymer Micelles for Therapeutic Agent Delivery.Curr Pharm Biotechnol. 2016;17(3):212-26. doi: 10.2174/1389201017666151223124135. Curr Pharm Biotechnol. 2016. PMID: 26696015 Review.
-
Controlled Self-Assembly of Amphiphilic Random Copolymers into Folded Micelles and Nanostructure Materials.J Oleo Sci. 2020 Jun 9;69(6):529-538. doi: 10.5650/jos.ess20089. Epub 2020 May 14. J Oleo Sci. 2020. PMID: 32404554 Review.
Cited by
-
Self-assembled 20-nm (64)Cu-micelles enhance accumulation in rat glioblastoma.J Control Release. 2015 Dec 28;220(Pt A):51-60. doi: 10.1016/j.jconrel.2015.09.057. Epub 2015 Oct 5. J Control Release. 2015. PMID: 26437259 Free PMC article.
-
Fabrication of self-assembling nanofibers with optimal cell uptake and therapeutic delivery efficacy.Bioact Mater. 2017 Sep 21;2(4):260-268. doi: 10.1016/j.bioactmat.2017.09.001. eCollection 2017 Dec. Bioact Mater. 2017. PMID: 29744435 Free PMC article.
-
Environment-dependent guest exchange in supramolecular hosts.Langmuir. 2014 Oct 21;30(41):12384-90. doi: 10.1021/la502760c. Epub 2014 Oct 6. Langmuir. 2014. PMID: 25244305 Free PMC article.
-
Molecular Simulations of PEGylated Biomolecules, Liposomes, and Nanoparticles for Drug Delivery Applications.Pharmaceutics. 2020 Jun 10;12(6):533. doi: 10.3390/pharmaceutics12060533. Pharmaceutics. 2020. PMID: 32531886 Free PMC article. Review.
-
Multicomponent peptide assemblies.Chem Soc Rev. 2018 May 21;47(10):3659-3720. doi: 10.1039/c8cs00115d. Chem Soc Rev. 2018. PMID: 29697126 Free PMC article. Review.
References
-
- Kataoka K, Harada A, Nagasaki Y. Adv Drug Delivery Rev. 2001;47:113–131. - PubMed
-
- Percec V, Wilson DA, Leowanawat P, Wilson CJ, Hughes AD, Kaucher MS, Hammer DA, Levine DH, Kim AJ, Bates FS, Davis KP, Lodge TP, Klein ML, DeVane RH, Aqad E, Rosen BM, Argintaru AO, Sienkowska MJ, Rissanen K, Nummelin S, Ropponen J. Science. 2010;328:1009–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

