Fetal microchimeric cells in a fetus-treats-its-mother paradigm do not contribute to dystrophin production in serially parous mdx females
- PMID: 22731493
- PMCID: PMC3464073
- DOI: 10.1089/scd.2012.0047
Fetal microchimeric cells in a fetus-treats-its-mother paradigm do not contribute to dystrophin production in serially parous mdx females
Abstract
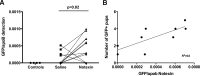
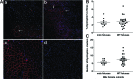
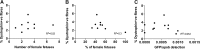
Throughout every pregnancy, genetically distinct fetal microchimeric stem/progenitor cells (FMCs) engraft in the mother, persist long after delivery, and may home to damaged maternal tissues. Phenotypically normal fetal lymphoid progenitors have been described to develop in immunodeficient mothers in a fetus-treats-its-mother paradigm. Since stem cells contribute to muscle repair, we assessed this paradigm in the mdx mouse model of Duchenne muscular dystrophy. mdx females were bred serially to either ROSAeGFP males or mdx males to obtain postpartum microchimeras that received either wild-type FMCs or dystrophin-deficient FMCs through serial gestations. To enhance regeneration, notexin was injected into the tibialis anterior of postpartum mice. FMCs were detected by qPCR at a higher frequency in injected compared to noninjected side muscle (P=0.02). However, the number of dystrophin-positive fibers was similar in mothers delivering wild-type compared to mdx pups. In addition, there was no correlation between FMC detection and percentage dystrophin, and no GFP+ve FMCs were identified that expressed dystrophin. In 10/11 animals, GFP+ve FMCs were detected by immunohistochemistry, of which 60% expressed CD45 with 96% outside the basal lamina defining myofiber contours. Finally we confirmed lack of FMC contribution to statellite cells in postpartum mdx females mated with Myf5-LacZ males. We conclude that the FMC contribution to regenerating muscles is insufficient to have a functional impact.
Figures


Similar articles
-
Alterations in Notch signalling in skeletal muscles from mdx and dko dystrophic mice and patients with Duchenne muscular dystrophy.Exp Physiol. 2014 Apr;99(4):675-87. doi: 10.1113/expphysiol.2013.077255. Epub 2014 Jan 17. Exp Physiol. 2014. PMID: 24443351
-
A highly functional mini-dystrophin/GFP fusion gene for cell and gene therapy studies of Duchenne muscular dystrophy.Hum Mol Genet. 2006 May 15;15(10):1610-22. doi: 10.1093/hmg/ddl082. Epub 2006 Apr 4. Hum Mol Genet. 2006. PMID: 16595609
-
Expression of a NOS transgene in dystrophin-deficient muscle reduces muscle membrane damage without increasing the expression of membrane-associated cytoskeletal proteins.Mol Genet Metab. 2004 Aug;82(4):312-20. doi: 10.1016/j.ymgme.2004.06.006. Mol Genet Metab. 2004. PMID: 15308129
-
Impaired regenerative capacity and lower revertant fibre expansion in dystrophin-deficient mdx muscles on DBA/2 background.Sci Rep. 2016 Dec 7;6:38371. doi: 10.1038/srep38371. Sci Rep. 2016. PMID: 27924830 Free PMC article.
-
Bone tissue and muscle dystrophin deficiency in mdx mice.Joint Bone Spine. 2012 Mar;79(2):129-33. doi: 10.1016/j.jbspin.2011.08.004. Epub 2011 Nov 12. Joint Bone Spine. 2012. PMID: 22079415 Review.
Cited by
-
Characterization of fetal microchimeric immune cells in mouse maternal hearts during physiologic and pathologic pregnancies.Front Cell Dev Biol. 2023 Sep 22;11:1256945. doi: 10.3389/fcell.2023.1256945. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37808080 Free PMC article.
-
Distant mesenchymal progenitors contribute to skin wound healing and produce collagen: evidence from a murine fetal microchimerism model.PLoS One. 2013 May 1;8(5):e62662. doi: 10.1371/journal.pone.0062662. Print 2013. PLoS One. 2013. PMID: 23650524 Free PMC article.
-
Forever Connected: The Lifelong Biological Consequences of Fetomaternal and Maternofetal Microchimerism.Clin Chem. 2021 Jan 30;67(2):351-362. doi: 10.1093/clinchem/hvaa304. Clin Chem. 2021. PMID: 33417673 Free PMC article. Review.
-
The when, what, and where of naturally-acquired microchimerism.Semin Immunopathol. 2025 Mar 11;47(1):20. doi: 10.1007/s00281-024-01029-2. Semin Immunopathol. 2025. PMID: 40067465 Review.
References
-
- Khosrotehrani K. Johnson KL. Cha DH. Salomon RN. Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292:75–80. - PubMed
-
- Khosrotehrani K. Leduc M. Bachy V. Nguyen Huu S. Oster M. Abbas A. Uzan S. Aractingi S. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and B cells in mothers. J Immunol. 2008;180:889–897. - PubMed
-
- Nassar D. Droitcourt C. Mathieu-d'Argent E. Kim MJ. Khosrotehrani K. Aractingi S. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J. 2012;26:149–157. - PubMed
-
- Nelson JL. Microchimerism and the causation of scleroderma. Scand J Rheumatol Suppl. 1998;107:10–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

