Gain-of-function analogues of the pore-forming peptide melittin selected by orthogonal high-throughput screening
- PMID: 22731650
- PMCID: PMC3443472
- DOI: 10.1021/ja3042004
Gain-of-function analogues of the pore-forming peptide melittin selected by orthogonal high-throughput screening
Abstract
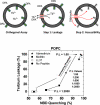
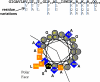
We recently developed an orthogonal, high-throughput assay to identify peptides that self-assemble into potent, equilibrium pores in synthetic lipid bilayers. Here, we use this assay as a high-throughput screen to select highly potent pore-forming peptides from a 7776-member rational combinatorial peptide library based on the sequence of the natural pore-forming peptide toxin melittin. In the library we varied ten critical residues in the melittin sequence, chosen to test specific structural hypotheses about the mechanism of pore formation. Using the new high-throughput assay, we screened the library for gain-of-function sequences at a peptide to lipid ratio of 1:1000 where native melittin is not active. More than 99% of the library sequences were also inactive under these conditions. A small number of library members (0.1%) were highly active. From these we identified 14 potent, gain-of-function, pore-forming sequences. These sequences differed from melittin in only 2-6 amino acids out of 26. Some native residues were highly conserved and others were consistently changed. The two factors that were essential for gain-of-function were the preservation of melittin's proline-dependent break in the middle of the helix and the improvement and extension the amphipathic nature of the α-helix. In particular the highly cationic carboxyl-terminal sequence of melittin, is consistently changed in the gain-of-function variants to a sequence that it is capable of participating in an extended amphipathic α-helix. The most potent variants reside in a membrane-spanning orientation, in contrast to the parent melittin, which is predominantly surface bound. This structural information, taken together with the high-throughput tools developed for this work, enable the identification, refinement and optimization of pore-forming peptides for many potential applications.
Figures



Similar articles
-
Synthetic molecular evolution of pore-forming peptides by iterative combinatorial library screening.ACS Chem Biol. 2013 Apr 19;8(4):823-31. doi: 10.1021/cb300598k. Epub 2013 Feb 20. ACS Chem Biol. 2013. PMID: 23394375 Free PMC article.
-
Determining the mechanism of membrane permeabilizing peptides: identification of potent, equilibrium pore-formers.Biochim Biophys Acta. 2012 Jul;1818(7):1625-32. doi: 10.1016/j.bbamem.2012.02.009. Biochim Biophys Acta. 2012. PMID: 22365969 Free PMC article.
-
The electrical response of bilayers to the bee venom toxin melittin: evidence for transient bilayer permeabilization.Biochim Biophys Acta. 2013 May;1828(5):1357-64. doi: 10.1016/j.bbamem.2013.01.021. Epub 2013 Feb 4. Biochim Biophys Acta. 2013. PMID: 23384418 Free PMC article.
-
Model Membrane and Cell Studies of Antimicrobial Activity of Melittin Analogues.Curr Top Med Chem. 2016;16(1):40-5. doi: 10.2174/1568026615666150703115919. Curr Top Med Chem. 2016. PMID: 26139117 Review.
-
The actions of melittin on membranes.Biochim Biophys Acta. 1990 May 7;1031(2):143-61. doi: 10.1016/0304-4157(90)90006-x. Biochim Biophys Acta. 1990. PMID: 2187536 Review.
Cited by
-
Mechanistic Landscape of Membrane-Permeabilizing Peptides.Chem Rev. 2019 May 8;119(9):6040-6085. doi: 10.1021/acs.chemrev.8b00520. Epub 2019 Jan 9. Chem Rev. 2019. PMID: 30624911 Free PMC article. Review.
-
Translocation of cationic amphipathic peptides across the membranes of pure phospholipid giant vesicles.J Am Chem Soc. 2013 Nov 6;135(44):16517-25. doi: 10.1021/ja407451c. Epub 2013 Oct 23. J Am Chem Soc. 2013. PMID: 24152283 Free PMC article.
-
Conformational Fine-Tuning of Pore-Forming Peptide Potency and Selectivity.J Am Chem Soc. 2015 Dec 30;137(51):16144-52. doi: 10.1021/jacs.5b10595. Epub 2015 Dec 18. J Am Chem Soc. 2015. PMID: 26632653 Free PMC article.
-
Liposome Deformation Induced by Membrane-Binding Peptides.Micromachines (Basel). 2023 Feb 2;14(2):373. doi: 10.3390/mi14020373. Micromachines (Basel). 2023. PMID: 36838073 Free PMC article.
-
Effects of Peptide Charge, Orientation, and Concentration on Melittin Transmembrane Pores.Biophys J. 2018 Jun 19;114(12):2865-2874. doi: 10.1016/j.bpj.2018.05.006. Biophys J. 2018. PMID: 29925023 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

