Effects of eszopiclone on safety, subjective measures of efficacy, and quality of life in elderly and nonelderly Japanese patients with chronic insomnia, both with and without comorbid psychiatric disorders: a 24-week, randomized, double-blind study
- PMID: 22731653
- PMCID: PMC3430596
- DOI: 10.1186/1744-859X-11-15
Effects of eszopiclone on safety, subjective measures of efficacy, and quality of life in elderly and nonelderly Japanese patients with chronic insomnia, both with and without comorbid psychiatric disorders: a 24-week, randomized, double-blind study
Abstract
Background: The primary objective of this study was to evaluate long-term (24-week) safety of eszopiclone in elderly and nonelderly Japanese patients with chronic insomnia. The secondary objectives were to evaluate short-term (4-week) efficacy and to assess for rebound insomnia or dependence after long-term treatment.
Methods: Patients (n = 164 elderly; n = 161 nonelderly), with or without psychiatric comorbidities, were randomized to receive low-dose (1 mg, elderly; 2 mg, nonelderly) or high-dose (2 mg, elderly; 3 mg, nonelderly) eszopiclone. The safety evaluation included adverse events, vital signs, clinical laboratory parameters, and electrocardiogram. Efficacy was assessed using patient reports of sleep latency (SL), total sleep time (TST), wake time after sleep onset (WASO), number of awakenings (NA), quality of sleep, depth of sleep, daytime sleepiness, daytime ability to function, and the 36-item Short Form (SF-36) Health Survey.
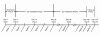
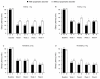
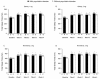
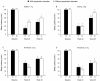
Results: The rate of adverse events was 81.5% in the 1-mg elderly group, 79.5% in the 2-mg elderly group, 82.1% in the 2-mg nonelderly group, and 87.0% in the 3-mg nonelderly group. Dysgeusia was the most common adverse event and was dose-related. Of 12 serious adverse events, none were considered by the investigator to be related to study medication. No rebound insomnia was observed. Eszopiclone significantly improved SL, TST, WASO, NA, and daytime sleepiness and function from baseline to Week 4, irrespective of age and psychiatric comorbidity. Improvements were also observed in SF-36 Mental Health Component scores in elderly and nonelderly patients with psychiatric comorbidities.
Conclusions: Irrespective of age, eszopiclone appeared safe as administered in this study for 24 weeks. Eszopiclone improved sleep variables in insomnia patients with and without psychiatric disorders and health-related quality of life in those with psychiatric disorders.
Trial registration: ClinicalTrials.gov #NCT00770692; http://clinicaltrials.gov/ct2/show/NCT00770692.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical

