Inhibition of protein-protein interaction of HER2-EGFR and HER2-HER3 by a rationally designed peptidomimetic
- PMID: 22731912
- PMCID: PMC3572747
- DOI: 10.1080/07391102.2012.687525
Inhibition of protein-protein interaction of HER2-EGFR and HER2-HER3 by a rationally designed peptidomimetic
Abstract
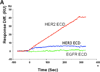
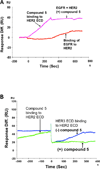
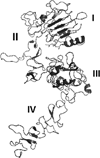
Protein-protein interactions (PPI) play a crucial role in many biological processes and modulation of PPI using small molecules to target hot spots has therapeutic value. As a model system we will use PPI of human epidermal growth factor receptors (EGFRs). Among the four EGFRs, EGFR-HER2 and HER2-HER3 are well known in cancer. We have designed a small molecule that is targeted to modulate HER2-mediated signaling. Our approach is novel because the small molecule designed disrupts dimerization not only of EGFR-HER2, but also of HER2-HER3. In the present study we have shown, using surface plasmon resonance analysis, that a peptidomimetic, compound 5, binds specifically to HER2 protein extracellular domain and disrupts the dimerization of EGFRs. To evaluate the effect of compound 5 on HER2 signaling in vitro, Western blot and PathHunter assays were used. Results indicated that compound 5 inhibits the phosphorylation of HER2 kinase domain and inhibits the heterodimerization in a dose-dependent manner. Molecular modeling methods were used to model the PPI of HER2-HER3 heterodimer.
Figures





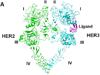

Similar articles
-
Design of novel lipidated peptidomimetic conjugates for targeting EGFR heterodimerization in HER2 + cancer.Bioorg Med Chem Lett. 2018 Dec 1;28(22):3506-3513. doi: 10.1016/j.bmcl.2018.10.005. Epub 2018 Oct 3. Bioorg Med Chem Lett. 2018. PMID: 30314880 Free PMC article.
-
Structure-activity relationships of peptidomimetics that inhibit PPI of HER2-HER3.Biopolymers. 2014 Jun;101(6):693-702. doi: 10.1002/bip.22441. Biopolymers. 2014. PMID: 24222531 Free PMC article.
-
A grafted peptidomimetic for EGFR heterodimerization inhibition: Implications in NSCLC models.Eur J Med Chem. 2021 Apr 15;216:113312. doi: 10.1016/j.ejmech.2021.113312. Epub 2021 Feb 23. Eur J Med Chem. 2021. PMID: 33667849 Free PMC article.
-
A role for the pseudokinase HER3 in the acquired resistance against EGFR- and HER2-directed targeted therapy.Biochem Soc Trans. 2014 Aug;42(4):831-6. doi: 10.1042/BST20140043. Biochem Soc Trans. 2014. PMID: 25109965 Review.
-
Interplay of the iron-regulated metastasis suppressor NDRG1 with epidermal growth factor receptor (EGFR) and oncogenic signaling.J Biol Chem. 2017 Aug 4;292(31):12772-12782. doi: 10.1074/jbc.R117.776393. Epub 2017 Jun 14. J Biol Chem. 2017. PMID: 28615452 Free PMC article. Review.
Cited by
-
Structure and flexibility of nanoscale protein cages designed by symmetric self-assembly.J Am Chem Soc. 2013 May 22;135(20):7738-43. doi: 10.1021/ja402277f. Epub 2013 May 8. J Am Chem Soc. 2013. PMID: 23621606 Free PMC article.
-
Design, Synthesis and Inhibitory Activity of Photoswitchable RET Kinase Inhibitors.Sci Rep. 2015 May 6;5:9769. doi: 10.1038/srep09769. Sci Rep. 2015. PMID: 25944708 Free PMC article.
-
Design of a doxorubicin-peptidomimetic conjugate that targets HER2-positive cancer cells.Eur J Med Chem. 2017 Jan 5;125:914-924. doi: 10.1016/j.ejmech.2016.10.015. Epub 2016 Oct 10. Eur J Med Chem. 2017. PMID: 27769032 Free PMC article.
-
Design, synthesis and characterization of peptidomimetic conjugate of BODIPY targeting HER2 protein extracellular domain.Eur J Med Chem. 2013 Jul;65:60-9. doi: 10.1016/j.ejmech.2013.04.038. Epub 2013 Apr 28. Eur J Med Chem. 2013. PMID: 23688700 Free PMC article.
-
Novel Peptidomimetics for Inhibition of HER2:HER3 Heterodimerization in HER2-Positive Breast Cancer.Chem Biol Drug Des. 2015 Jun;85(6):702-714. doi: 10.1111/cbdd.12453. Epub 2014 Nov 6. Chem Biol Drug Des. 2015. PMID: 25346057 Free PMC article.
References
-
- Ahn ER, Vogel CL. Dual HER2-targeted approaches in HER2-positive breast cancer. Breast Cancer Research and Treatment. 2011;131:371–383. - PubMed
-
- Allen S, Garrett JT, Rawale SV, Jones AL, Philips G, Forni G, Morris JC, Oshima RG, Kaumaya TP. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. Journal of Immunology. 2007;179:472–482. - PubMed
-
- Arcangeli C, Cantale C, Galeffi P, Gianese G, Paparcone R, Rosato V. Understanding structural/functional properties of immunoconjugates for cancer therapy by computational approaches. Journal of Biomolecular Structure and Dynamics. 2008;26:35–48. - PubMed
-
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL Workspace: A web-based environment for protein structure homology modeling. Bioinformatics. 2010;22:195–201. - PubMed
-
- Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L, Placido SD, Osborne KC, Schiff R. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. Journal of the National Cancer Institute. 2007;99:694–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
