Breakingtheice: a protocol for a randomised controlled trial of an internet-based intervention addressing amphetamine-type stimulant use
- PMID: 22731926
- PMCID: PMC3464697
- DOI: 10.1186/1471-244X-12-67
Breakingtheice: a protocol for a randomised controlled trial of an internet-based intervention addressing amphetamine-type stimulant use
Abstract
Background: The prevalence of amphetamine-type stimulant use is greater than that of opioids and cocaine combined. Currently, there are no approved pharmacotherapy treatments for amphetamine-type stimulant problems, but some face-to-face psychotherapies are of demonstrated effectiveness. However, most treatment services focus on alcohol or opioid disorders, have limited reach and may not appeal to users of amphetamine-type stimulants. Internet interventions have proven to be effective for some substance use problems but none has specifically targeted users of amphetamine-type stimulants.
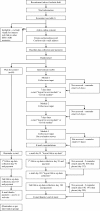
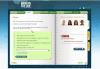
Design/method: The study will use a randomized controlled trial design to evaluate the effect of an internet intervention for amphetamine-type stimulant problems compared with a waitlist control group. The primary outcome will be assessed as amphetamine-type stimulant use (baseline, 3 and 6 months). Other outcomes measures will include 'readiness to change', quality of life, psychological distress (K-10 score), days out of role, poly-drug use, help-seeking intention and help-seeking behavior. The intervention consists of three modules requiring an estimated total completion time of 90 minutes. The content of the modules was adapted from face-to-face clinical techniques based on cognitive behavior therapy and motivation enhancement. The target sample is 160 men and women aged 18 and over who have used amphetamine-type stimulants in the last 3 months.
Discussion: To our knowledge this will be the first randomized controlled trial of an internet intervention specifically developed for users of amphetamine-type stimulants. If successful, the intervention will offer greater reach than conventional therapies and may engage clients who do not generally seek treatment from existing service providers.
Trial registration: Australian and New Zealand Clinical Trials Registry (http://www.anzctr.org.au/) ACTRN12611000947909.
Figures
References
-
- Baker A, Lee NK, Jenner L. Models of intervention and care for psychostimulant users. 2. Australian Government Department of Health and Ageing, Canberra; 2004.
-
- Drug Strategy Ministerial Council on. National Amphetamine-Type Stimulant Strategy 2008-2011. [ http://www.nationaldrugstrategy.gov.au/internet/drugstrategy/publishing....]
-
- United Nations Office of Drugs and Crime. World Drug Report: Volume 1 Analysis. United Nations Publication, Vienna; 2006.
-
- United Nations Office of Drugs and Crime. Patterns and Trends of Amphetamine-Type Stimulants and other Drugs. Asia and the Pacific, Vienna; 2010. pp. 1–158.
-
- Australian Institute of Health and Welfare. 2010 National Drug Strategy Household Survey. AIHW, Canberra; 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials