Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae
- PMID: 22731941
- PMCID: PMC3414772
- DOI: 10.1186/1741-7007-10-59
Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae
Abstract
Background: The nematode Caenorhabditis elegans is a major model organism in laboratory biology. Very little is known, however, about its ecology, including where it proliferates. In the past, C. elegans was mainly isolated from human-made compost heaps, where it was overwhelmingly found in the non-feeding dauer diapause stage.
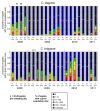
Results: C. elegans and C. briggsae were found in large, proliferating populations in rotting plant material (fruits and stems) in several locations in mainland France. Both species were found to co-occur in samples isolated from a given plant species. Population counts spanned a range from one to more than 10,000 Caenorhabditis individuals on a single fruit or stem. Some populations with an intermediate census size (10 to 1,000) contained no dauer larvae at all, whereas larger populations always included some larvae in the pre-dauer or dauer stages. We report on associated micro-organisms, including pathogens. We systematically sampled a spatio-temporally structured set of rotting apples in an apple orchard in Orsay over four years. C. elegans and C. briggsae were abundantly found every year, but their temporal distributions did not coincide. C. briggsae was found alone in summer, whereas both species co-occurred in early fall and C. elegans was found alone in late fall. Competition experiments in the laboratory at different temperatures show that C. briggsae out-competes C. elegans at high temperatures, whereas C. elegans out-competes C. briggsae at lower temperatures.
Conclusions: C. elegans and C. briggsae proliferate in the same rotting vegetal substrates. In contrast to previous surveys of populations in compost heaps, we found fully proliferating populations with no dauer larvae. The temporal sharing of the habitat by the two species coincides with their temperature preference in the laboratory, with C. briggsae populations growing faster than C. elegans at higher temperatures, and vice at lower temperatures.
Figures


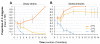
Comment in
-
The worm in the world and the world in the worm.BMC Biol. 2012 Jun 25;10:57. doi: 10.1186/1741-7007-10-57. BMC Biol. 2012. PMID: 22731915 Free PMC article.
References
-
- Riddle DL. In: The Nematode Caenorhabditis elegans. Wood WB, editor. Cold Spring Harbor: Cold Spring Harbor Press; 1988. The dauer larva; pp. 393–412.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

