A case series of low dose bevacizumab and chemotherapy in heavily pretreated patients with epithelial ovarian cancer
- PMID: 22732001
- PMCID: PMC3408333
- DOI: 10.1186/1757-2215-5-17
A case series of low dose bevacizumab and chemotherapy in heavily pretreated patients with epithelial ovarian cancer
Abstract
Background: The addition of bevacizumab to standard chemotherapy prolongs progression free survival in the first line treatment of epithelial ovarian cancer (EOC), but its cost/effectiveness is debated. We assessed the safety and activity of a lower dose of bevacizumab in pretreated advanced stage EOC.
Methods: We treated 15 patients, mostly with platinum resistant EOC, who had received a median of four prior cytotoxic regimens, with bevacizumab 5-7.5 mg/kg q21 days in combination with either carboplatin (n = 8), oral cyclofosfamide (n = 5) or weekly paclitaxel (n = 2). Bevacizumab was administered until disease progression. Tumor response was assessed by CA125 and fusion 18 F-FDG PET/contrast enhanced CT.
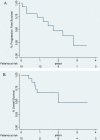
Results: The median number of bevacizumab cycles was 21 (range 3-59). The median baseline CA125 was 272 U/ml and decreased to 15.2 U/ml at nadir. Tumor response was 4 complete response (CR) (26.7%) and 7 partial response (PR) (46.7%) by chemotherapy (CT), with an overall response rate of 73.4% (95% CI, 51.0 - 95.8) according to Response Evaluation Criteria In Solid Tumors (RECIST), and 6 CR (40%) and 4 PR (26.7%) by PET, for an overall metabolic response rate of 67% (95%CI, 42.8 - 90.6) according to PET Response Criteria in Solid Tumors (PERCIST). Median progression free survival (PFS) was 21 months and median overall survival (OS) was 24 months. Grade 3 adverse events related to bevacizumab were hypertension (n = 2), proteinuria (n = 1) and epistaxis (n = 5). Treatment was delayed in five patients for nasal bleeding or uncontrolled hypertension.
Conclusions: Low-dose bevacizumab and chemotherapy was well tolerated and active in a heavily pretreated population of advanced EOC. Further studies should assess the activity of low dose bevacizumab in EOC.
Figures


References
-
- Hiclin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. - PubMed
-
- Shimogai R, Kigawa J, Itamochi H, Iba T, Kanamori Y, Oishi T, Shimada M, Sato S, Kawaguchi W, Sato S, Terakawa N. Expression of hypoxia-inducible factor 1alha gene affects the outcome in patients with ovarian cancer. Int J Gynecol Cancer. 2008;18:499–505. doi: 10.1111/j.1525-1438.2007.01055.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

