Central and overlapping role of Cathepsin B and inflammasome adaptor ASC in antigen presenting function of human dendritic cells
- PMID: 22732093
- PMCID: PMC4084975
- DOI: 10.1016/j.humimm.2012.06.008
Central and overlapping role of Cathepsin B and inflammasome adaptor ASC in antigen presenting function of human dendritic cells
Abstract
Inflammasomes are increasingly implicated in regulating immunity, but how their activation relates to function of human dendritic cells (DCs) is unknown. Here we show that DC maturation stimuli lead to rapid activation of caspase-1 in human monocyte-derived DCs. RNAi mediated inhibition of the inflammasome component ASC leads to marked inhibition of the capacity of lipopolysachharide (LPS)-matured DCs to stimulate antigen-specific T cells. RNAi mediated inhibition of Cathepsin B (CatB) also similarly inhibits the capacity of human DCs to stimulate immunity. The defective ability of ASC or CatB deficient DCs to stimulate T cells is independent of inflammasome-mediated processing of inflammatory cytokines and also includes DCs loaded with pre-processed peptide. Gene expression profiles of ASC or CatB deficient human DCs show marked overlap with downregulation of genes implicated in DC function. These data demonstrate an important role for ASC and CatB in regulating function of human DCs with overlapping effects on gene expression.
Copyright © 2012 American Society for Histocompatibility and Immunogenetics. Published by Elsevier Inc. All rights reserved.
Figures
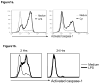
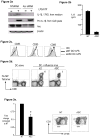
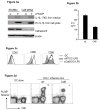
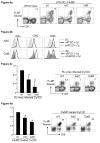

References
-
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419. - PubMed
-
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821. - PubMed
-
- Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299(5611):1400. - PubMed
-
- Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21(2):155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

