Parametric studies of antipsychotic-induced sensitization in the conditioned avoidance response model: roles of number of drug exposure, drug dose, and test-retest interval
- PMID: 22732209
- PMCID: PMC4756434
- DOI: 10.1097/FBP.0b013e32835651ea
Parametric studies of antipsychotic-induced sensitization in the conditioned avoidance response model: roles of number of drug exposure, drug dose, and test-retest interval
Abstract
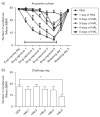
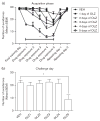
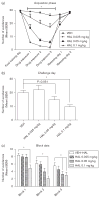
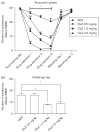
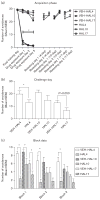
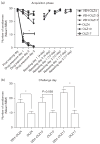
Repeated haloperidol and olanzapine treatment produces an enhanced disruption of avoidance responding, a validated measure of antipsychotic activity. Experimental parameters affecting this sensitization-like effect have not been thoroughly examined. The present study investigated the role of three parameters (number of injections, dose, and interval between initial exposure and challenge) in antipsychotic sensitization in the conditioned avoidance response paradigm. Well-trained Sprague-Dawley rats received different numbers of drug treatment (1-5 days) or different doses of haloperidol (0.025-0.10 mg/kg, subcutaneously) or olanzapine (0.5-2.0 mg/kg, subcutaneously). After certain time intervals (4, 10 or 17 days), they were tested for the expression of haloperidol or olanzapine sensitization in a challenge test in which all rats were injected with a lower dose of haloperidol (0.025 mg/kg) or olanzapine (0.5 mg/kg). Throughout the drug-treatment period, both haloperidol and olanzapine dose-dependently enhanced their disruption of avoidance responding. Three days later, the sensitization induced by a low dose of haloperidol (0.025 mg/kg) or olanzapine (0.5 mg/kg) was only apparent in rats that received treatment for 5 days, but not in those that received treatment for 1-4 days. The sensitization induced by the medium and high doses of haloperidol (0.05 and 0.10 mg/kg) or olanzapine (1.0 and 2.0 mg/kg) was still robust even with only 3 days of treatment. The sensitization induced by a 3-day haloperidol (0.10 mg/kg) and olanzapine (2.0 mg/kg) treatment was long-lasting, still detectable 17 days after the last drug treatment. This study suggests that antipsychotic sensitization is a robust behavioral phenomenon. Its induction and expression are strongly influenced by parameters such as number of drug exposures, drug dose, and test-retest interval. Given the importance of antipsychotic sensitization in the maintenance of antipsychotic effects in the clinic, this study introduces a paradigm that can be used to investigate the behavioral and neurobiological mechanisms underlying antipsychotic sensitization.
Conflict of interest statement
There are no conflicts of interest.
Figures


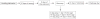
Similar articles
-
Contextual and behavioral control of antipsychotic sensitization induced by haloperidol and olanzapine.Behav Pharmacol. 2012 Feb;23(1):66-79. doi: 10.1097/FBP.0b013e32834ecac4. Behav Pharmacol. 2012. PMID: 22157143 Free PMC article.
-
An investigation of the behavioral mechanisms of antipsychotic action using a drug-drug conditioning paradigm.Behav Pharmacol. 2009 Mar;20(2):184-94. doi: 10.1097/FBP.0b013e32832a8f66. Behav Pharmacol. 2009. PMID: 19322074 Free PMC article.
-
Drug-drug conditioning between citalopram and haloperidol or olanzapine in a conditioned avoidance response model: implications for polypharmacy in schizophrenia.Behav Pharmacol. 2012 Oct;23(7):658-68. doi: 10.1097/FBP.0b013e328358590d. Behav Pharmacol. 2012. PMID: 22903071 Free PMC article.
-
[Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation].Encephale. 2005 Mar-Apr;31(2):235-46. doi: 10.1016/s0013-7006(05)82390-5. Encephale. 2005. PMID: 15959450 Review. French.
-
Behavioral pharmacology of olanzapine: a novel antipsychotic drug.J Clin Psychiatry. 1997;58 Suppl 10:37-44. J Clin Psychiatry. 1997. PMID: 9265915 Review.
Cited by
-
Repeated administration of aripiprazole produces a sensitization effect in the suppression of avoidance responding and phencyclidine-induced hyperlocomotion and increases D2 receptor-mediated behavioral function.J Psychopharmacol. 2015 Apr;29(4):390-400. doi: 10.1177/0269881114565937. Epub 2015 Jan 13. J Psychopharmacol. 2015. PMID: 25586399 Free PMC article.
-
Environmental and behavioral controls of the expression of clozapine tolerance: evidence from a novel across-model transfer paradigm.Behav Brain Res. 2013 Feb 1;238:178-87. doi: 10.1016/j.bbr.2012.10.009. Epub 2012 Oct 23. Behav Brain Res. 2013. PMID: 23092709 Free PMC article.
-
Repeated effects of the neurotensin receptor agonist PD149163 in three animal tests of antipsychotic activity: assessing for tolerance and cross-tolerance to clozapine.Pharmacol Biochem Behav. 2015 Jan;128:78-88. doi: 10.1016/j.pbb.2014.11.015. Epub 2014 Nov 26. Pharmacol Biochem Behav. 2015. PMID: 25433325 Free PMC article.
-
Continuous oral olanzapine or clozapine treatment initiated in adolescence has differential short- and long-term impacts on antipsychotic sensitivity than those initiated in adulthood.Eur J Pharmacol. 2024 Jun 5;972:176567. doi: 10.1016/j.ejphar.2024.176567. Epub 2024 Apr 4. Eur J Pharmacol. 2024. PMID: 38582275 Free PMC article.
-
Repeated asenapine treatment produces a sensitization effect in two preclinical tests of antipsychotic activity.Neuropharmacology. 2013 Dec;75:356-64. doi: 10.1016/j.neuropharm.2013.05.031. Epub 2013 Aug 14. Neuropharmacology. 2013. PMID: 23954676 Free PMC article.
References
-
- Amtage J, Schmidt WJ. Context-dependent catalepsy intensification is due to classical conditioning and sensitization. Behav Pharmacol. 2003;14:563–567. - PubMed
-
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. - PubMed
-
- Antelman SM, Gershon S. Clinical application of time-dependent sensitization to antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:65–78. - PubMed
-
- Antelman SM, Levine J, Gershon S. Time-dependent sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. Mol Psychiatry. 2000;5:350–356. - PubMed
-
- Barnes DE, Robinson B, Csernansky JG, Bellows EP. Sensitization versus tolerance to haloperidol-induced catalepsy: multiple determinants. Pharmacol Biochem Behav. 1990;36:883–887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

