Calcium-activated chloride channels in the apical region of mouse vomeronasal sensory neurons
- PMID: 22732308
- PMCID: PMC3382724
- DOI: 10.1085/jgp.201210780
Calcium-activated chloride channels in the apical region of mouse vomeronasal sensory neurons
Abstract
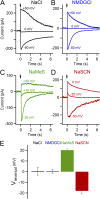
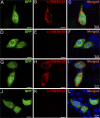
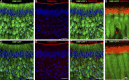
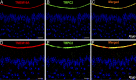
The rodent vomeronasal organ plays a crucial role in several social behaviors. Detection of pheromones or other emitted signaling molecules occurs in the dendritic microvilli of vomeronasal sensory neurons, where the binding of molecules to vomeronasal receptors leads to the influx of sodium and calcium ions mainly through the transient receptor potential canonical 2 (TRPC2) channel. To investigate the physiological role played by the increase in intracellular calcium concentration in the apical region of these neurons, we produced localized, rapid, and reproducible increases in calcium concentration with flash photolysis of caged calcium and measured calcium-activated currents with the whole cell voltage-clamp technique. On average, a large inward calcium-activated current of -261 pA was measured at -50 mV, rising with a time constant of 13 ms. Ion substitution experiments showed that this current is anion selective. Moreover, the chloride channel blockers niflumic acid and 4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid partially inhibited the calcium-activated current. These results directly demonstrate that a large chloride current can be activated by calcium in the apical region of mouse vomeronasal sensory neurons. Furthermore, we showed by immunohistochemistry that the calcium-activated chloride channels TMEM16A/anoctamin1 and TMEM16B/anoctamin2 are present in the apical layer of the vomeronasal epithelium, where they largely colocalize with the TRPC2 transduction channel. Immunocytochemistry on isolated vomeronasal sensory neurons showed that TMEM16A and TMEM16B coexpress in the neuronal microvilli. Therefore, we conclude that microvilli of mouse vomeronasal sensory neurons have a high density of calcium-activated chloride channels that may play an important role in vomeronasal transduction.
Figures





Similar articles
-
Conditional knockout of TMEM16A/anoctamin1 abolishes the calcium-activated chloride current in mouse vomeronasal sensory neurons.J Gen Physiol. 2015 Apr;145(4):285-301. doi: 10.1085/jgp.201411348. Epub 2015 Mar 16. J Gen Physiol. 2015. PMID: 25779870 Free PMC article.
-
Calcium concentration jumps reveal dynamic ion selectivity of calcium-activated chloride currents in mouse olfactory sensory neurons and TMEM16b-transfected HEK 293T cells.J Physiol. 2010 Nov 1;588(Pt 21):4189-204. doi: 10.1113/jphysiol.2010.194407. Epub 2010 Sep 13. J Physiol. 2010. PMID: 20837642 Free PMC article.
-
Ca2+-activated Cl- currents in the murine vomeronasal organ enhance neuronal spiking but are dispensable for male-male aggression.J Biol Chem. 2018 Jun 29;293(26):10392-10403. doi: 10.1074/jbc.RA118.003153. Epub 2018 May 16. J Biol Chem. 2018. PMID: 29769308 Free PMC article.
-
The TRPC2 ion channel and pheromone sensing in the accessory olfactory system.Naunyn Schmiedebergs Arch Pharmacol. 2005 Apr;371(4):245-50. doi: 10.1007/s00210-005-1028-8. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15871013 Review.
-
The physiological roles of anoctamin2/TMEM16B and anoctamin1/TMEM16A in chemical senses.Cell Calcium. 2024 Jun;120:102889. doi: 10.1016/j.ceca.2024.102889. Epub 2024 Apr 18. Cell Calcium. 2024. PMID: 38677213 Review.
Cited by
-
Ca2+-Activated Ion Channels Exert Opposite Effects in Different Signaling Compartments of Vomeronasal Sensory Neurons.J Neurosci. 2025 Apr 16;45(16):e2134242025. doi: 10.1523/JNEUROSCI.2134-24.2025. J Neurosci. 2025. PMID: 40032527 Free PMC article.
-
Conditional knockout of TMEM16A/anoctamin1 abolishes the calcium-activated chloride current in mouse vomeronasal sensory neurons.J Gen Physiol. 2015 Apr;145(4):285-301. doi: 10.1085/jgp.201411348. Epub 2015 Mar 16. J Gen Physiol. 2015. PMID: 25779870 Free PMC article.
-
Calmodulin-dependent activation and inactivation of anoctamin calcium-gated chloride channels.J Gen Physiol. 2013 Oct;142(4):381-404. doi: 10.1085/jgp.201311015. J Gen Physiol. 2013. PMID: 24081981 Free PMC article.
-
Tracking of unfamiliar odors is facilitated by signal amplification through anoctamin 2 chloride channels in mouse olfactory receptor neurons.Physiol Rep. 2017 Aug;5(15):e13373. doi: 10.14814/phy2.13373. Physiol Rep. 2017. PMID: 28784854 Free PMC article.
-
Developmental Role of Anoctamin-1/TMEM16A in Ca(2+)-Dependent Volume Change in Supporting Cells of the Mouse Cochlea.Exp Neurobiol. 2013 Dec;22(4):322-9. doi: 10.5607/en.2013.22.4.322. Epub 2013 Dec 31. Exp Neurobiol. 2013. PMID: 24465148 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

