Quantification of CD4 responses to combined antiretroviral therapy over 5 years among HIV-infected children in Kinshasa, Democratic Republic of Congo
- PMID: 22732464
- PMCID: PMC5592966
- DOI: 10.1097/QAI.0b013e31825bd9b7
Quantification of CD4 responses to combined antiretroviral therapy over 5 years among HIV-infected children in Kinshasa, Democratic Republic of Congo
Abstract
Background: The long-term effects of combined antiretroviral therapy (cART) on CD4 percentage in HIV-infected children are incompletely understood, with evidence from resource-deprived areas particularly scarce even though most children with HIV live in such settings. We sought to describe this relationship.
Methods: Observational longitudinal data from cART-naive children enrolled between December 2004 and May 2010 into an HIV care and treatment program in Kinshasa, Democratic Republic of Congo were analyzed. To estimate the effect of cART on CD4 percentage while accounting for time-dependent confounders affected by prior exposure to cART, a marginal structural linear mean model was used.
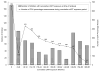
Results: Seven hundred ninety children were active for 2090 person-years and a median of 31 months; 619 (78%) initiated cART. At baseline, 405 children (51%) were in HIV clinical stage 3 or 4; 528 (67%) had advanced or severe immunodeficiency. Compared with no cART, the estimated absolute rise in CD4 percentage was 6.8% [95% confidence interval (CI), 4.7% to 8.9%] after 6 months of cART, 8.6% (95% CI, 7.0% to 10.2%) after 12 months, and 20.5% (95% CI, 16.1% to 24.9%) after 60 months. cART-mediated CD4 percentage gains were slowest but greatest among children with baseline CD4 percentage <15. The cumulative incidence of recovery to "not significant" World Health Organization age-specific immunodeficiency was lower if cART was started when immunodeficiency was severe rather than mild or advanced.
Conclusions: cART increased CD4 percentages among HIV-infected children in a resource-deprived setting, as previously noted among children in the United States. More gradual and protracted recovery in children with lower baseline CD4 percentages supports earlier initiation of pediatric cART.
Conflict of interest statement
The authors have no conflicts of interest to disclose. However, Drs Edmonds, Yotebieng, Lusiama, Matumona, Kitetele, Van Rie, and Behets declared that money was paid to their institution, in grants, for the HIV care and treatment program that provided the data for this study (grants were not for this specific secondary data analysis; rather, they were for the “parent” program). Dr Napravnik has stated that her institution has received grant support from Pfizer, Bristol-Myers Squibb, and Merck (these financial activities are outside of the submitted work).
Figures


Similar articles
-
The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study.PLoS Med. 2011 Jun;8(6):e1001044. doi: 10.1371/journal.pmed.1001044. Epub 2011 Jun 14. PLoS Med. 2011. PMID: 21695087 Free PMC article.
-
Predicting mortality in HIV-infected children initiating highly active antiretroviral therapy in a resource-deprived setting.Pediatr Infect Dis J. 2014 Nov;33(11):1148-55. doi: 10.1097/INF.0000000000000454. Pediatr Infect Dis J. 2014. PMID: 24945879
-
Implementation and Operational Research: Maternal Combination Antiretroviral Therapy Is Associated With Improved Retention of HIV-Exposed Infants in Kinshasa, Democratic Republic of Congo.J Acquir Immune Defic Syndr. 2015 Jul 1;69(3):e93-9. doi: 10.1097/QAI.0000000000000644. J Acquir Immune Defic Syndr. 2015. PMID: 25886922 Free PMC article.
-
Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy?BMC Med. 2013 Nov 27;11:251. doi: 10.1186/1741-7015-11-251. BMC Med. 2013. PMID: 24283830 Free PMC article. Review.
-
Influence of Combination Antiretroviral Therapy on HIV-1 Serological Responses and Their Implications: A Systematic Review and Meta-Analysis.Front Immunol. 2022 Mar 30;13:844023. doi: 10.3389/fimmu.2022.844023. eCollection 2022. Front Immunol. 2022. PMID: 35432309 Free PMC article.
Cited by
-
Regulatory T Cells Kinetics in Immune Reconstitution Inflammatory Syndrome in HIV-Tuberculosis Co-Infected Individuals.J Mol Clin Med. 2025;8(1):25012. doi: 10.31083/JMCM25012. Epub 2025 Feb 5. J Mol Clin Med. 2025. PMID: 40264927 Free PMC article.
-
Early Antiretroviral Therapy Initiation and Mortality Among Infants Diagnosed With HIV in the First 12 Weeks of Life: Experiences From Kinshasa, DR Congo and Blantyre, Malawi.Pediatr Infect Dis J. 2017 Jul;36(7):654-658. doi: 10.1097/INF.0000000000001539. Pediatr Infect Dis J. 2017. PMID: 28060044 Free PMC article.
-
Global temporal changes in the proportion of children with advanced disease at the start of combination antiretroviral therapy in an era of changing criteria for treatment initiation.J Int AIDS Soc. 2018 Nov;21(11):e25200. doi: 10.1002/jia2.25200. J Int AIDS Soc. 2018. PMID: 30614622 Free PMC article.
-
Continuous quality improvement interventions to improve long-term outcomes of antiretroviral therapy in women who initiated therapy during pregnancy or breastfeeding in the Democratic Republic of Congo: design of an open-label, parallel, group randomized trial.BMC Health Serv Res. 2017 Apr 26;17(1):306. doi: 10.1186/s12913-017-2253-9. BMC Health Serv Res. 2017. PMID: 28446232 Free PMC article. Clinical Trial.
-
Using CD4 percentage and age to optimize pediatric antiretroviral therapy initiation.Pediatrics. 2014 Oct;134(4):e1104-16. doi: 10.1542/peds.2014-0527. Pediatrics. 2014. PMID: 25266426 Free PMC article. Clinical Trial.
References
-
- Fahey JL, Prince H, Weaver M, et al. Quantitative changes in T helper or T suppressor/cytotoxic lymphocyte subsets that distinguish acquired immune deficiency syndrome from other immune subset disorders. Am J Med. 1984;76:95–100. - PubMed
-
- Fauci AS, Macher AM, Longo DL, et al. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. - PubMed
-
- Nikolic-Djokic D, Essajee S, Rigaud M, et al. Immunoreconstitution in children receiving highly active antiretroviral therapy depends on the CD4 cell percentage at baseline. J Infect Dis. 2002;185:290–298. - PubMed
-
- Soh CH, Oleske JM, Brady MT, et al. Long-term effects of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet. 2003;362:2045–2051. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

