Helminth infection impairs autophagy-mediated killing of bacterial enteropathogens by macrophages
- PMID: 22732589
- PMCID: PMC3423331
- DOI: 10.4049/jimmunol.1200484
Helminth infection impairs autophagy-mediated killing of bacterial enteropathogens by macrophages
Abstract
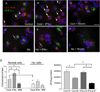
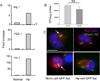
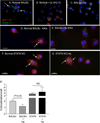
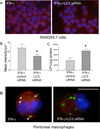
Autophagy is an important mechanism used by macrophages to kill intracellular pathogens. The results reported in this study demonstrate that autophagy is also involved in the macrophage killing of the extracellular enteropathogen Citrobacter rodentium after phagocytosis. The process was significantly impaired in macrophages isolated from mice chronically infected with the helminth parasite Heligmosomoides polygyrus. The H. polygyrus-mediated inhibition of autophagy was Th2 dependent because it was not observed in macrophages isolated from helminth-infected STAT6-deficient mice. Moreover, autophagy of Citrobacter was inhibited by treating macrophages with IL-4 and IL-13. The effect of H. polygyrus on autophagy was associated with decreased expression and processing of L chain protein 3 (LC3), a key component of the autophagic machinery. The helminth-induced inhibition of LC3 expression and processing was STAT6 dependent and could be recapitulated by treatment of macrophages with IL-4 and IL-13. Knockdown of LC3 significantly inhibited autophagic killing of Citrobacter, attesting to the functional importance of the H. polygyrus-mediated downregulation of this process. These observations reveal a new aspect of the immunosuppressive effects of helminth infection and provide mechanistic insights into our earlier finding that H. polygyrus significantly worsens the in vivo course of Citrobacter infection.
Figures
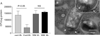

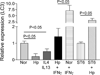
References
-
- Nokes C, Cooper E, Robinson B, Bundy D. Geohelminth infection and academic assessment in Jamaican children. Trans R Soc Trop Med Hyg. 1991;2:272–273. - PubMed
-
- Crompton D, Nesheim M. Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition. 2002;22:35–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

