Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity
- PMID: 22732595
- PMCID: PMC3401327
- DOI: 10.4049/jimmunol.1200633
Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity
Abstract
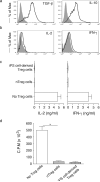
Regulatory T (Treg) cells are being used to treat autoimmunity and prevent organ rejection; however, Treg cell-based therapies have been hampered by the technical limitation in obtaining a high number of functional Treg cells. In this study, we show how to generate functional Treg cells from induced pluripotent stem (iPS) cells and to determine the potential role of such cells for Treg cell-based immunotherapy against autoimmunity in a therapeutic setting. Ligation of a Notch ligand and transduction of the gene Foxp3 induce iPS cells to differentiate into Treg cells. Expression of Foxp3 and coculture on Notch ligand-expressing stromal cells augment expression of CD3, TCR, CD4, CD25, and CTLA-4 on iPS cell-differentiated Treg cells, which are able to secrete TGF-β and IL-10 both in vivo and in vitro. Importantly, adoptive transfer of iPS cell-derived Treg cells expressing large amounts of Foxp3 and Bcl-x(L) significantly suppresses host immune responses and reduces arthritis development within murine models. These data suggest that Notch signaling and Foxp3 regulate the development and function of Treg cells derived from iPS cells. Our results provide a novel approach for generating potentially therapeutic Treg cells for the treatment of autoimmune diseases.
Figures


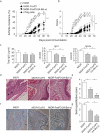
 ) in HE staining and massive destruction of cartilage (
) in HE staining and massive destruction of cartilage ( ) in Safranin O-fast green staining are indicated. Data are representative of three similar experiments (*P <0.05, one-way ANOVA tests).
) in Safranin O-fast green staining are indicated. Data are representative of three similar experiments (*P <0.05, one-way ANOVA tests).
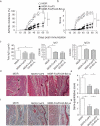
 ) in HE staining and massive destruction of cartilage (
) in HE staining and massive destruction of cartilage ( ) in Safranin O-fast green staining are indicated. Data are representative of three independent experiments.
) in Safranin O-fast green staining are indicated. Data are representative of three independent experiments.
References
-
- Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, Teng B, Holt GE, Standifer NE, Braun KR, Xie CF, Samuels PL, Vernon RB, Gebe JA, Wight TN, Nepom GT. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci U S A. 108:7938–7943. - PMC - PubMed
-
- Fritzsching B, Oberle N, Eberhardt N, Quick S, Haas J, Wildemann B, Krammer PH, Suri-Payer E. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand- but not to TCR-mediated cell death. J Immunol. 2005;175:32–36. - PubMed
-
- Li R, Perez N, Karumuthil-Melethil S, Prabhakar BS, Holterman MJ, Vasu C. Enhanced engagement of CTLA-4 induces antigen-specific CD4+CD25+Foxp3+ and CD4+CD25- TGF-beta 1+ adaptive regulatory T cells. J Immunol. 2007;179:5191–5203. - PubMed
-
- Van VQ, Darwiche J, Raymond M, Lesage S, Bouguermouh S, Rubio M, Sarfati M. Cutting edge: CD47 controls the in vivo proliferation and homeostasis of peripheral CD4+ CD25+ Foxp3+ regulatory T cells that express CD103. J Immunol. 2008;181:5204–5208. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

