Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling
- PMID: 22732731
- PMCID: PMC3458182
- DOI: 10.1053/j.gastro.2012.06.034
Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling
Abstract
Background & aims: Altered gastrointestinal motility is associated with significant morbidity and health care costs. Toll-like receptors (TLR) regulate intestinal homeostasis. We examined the roles of TLR4 signaling in survival of enteric neurons and gastrointestinal motility.
Methods: We assessed changes in intestinal motility by assessing stool frequency, bead expulsion, and isometric muscle recordings of colonic longitudinal muscle strips from mice that do not express TLR4 (Tlr4(Lps-d) or TLR4(-/-)) or Myd88 (Myd88(-/-)), in wild-type germ-free mice or wild-type mice depleted of the microbiota, and in mice with neural crest-specific deletion of Myd88 (Wnt1Cre(+/-)/Myd88(fl/fl)). We studied the effects of the TLR4 agonist lipopolysaccharide (LPS) on survival of cultured, immortalized fetal enteric neurons and enteric neuronal cells isolated from wild-type and Tlr4(Lps-d) mice at embryonic day 13.5.
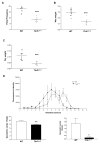
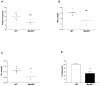
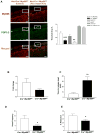
Results: There was a significant delay in gastrointestinal motility and reduced numbers of nitrergic neurons in TLR4(Lps-d), TLR4(-/-), and Myd88(-/-) mice compared with wild-type mice. A similar phenotype was observed in germ-free mice, mice depleted of intestinal microbiota, and Wnt1Cre(+/-)/Myd88(fl/fl) mice. Incubation of enteric neuronal cells with LPS led to activation of the transcription factor nuclear factor (NF)-κB and increased cell survival.
Conclusions: Interactions between enteric neurons and microbes increases neuron survival and gastrointestinal motility in mice. LPS activation of TLR4 and NF-κB appears to promote survival of enteric neurons. Factors that regulate TLR4 signaling in neurons might be developed to alter gastrointestinal motility.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. - PubMed
-
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. - PubMed
-
- van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res. 2009;175:139–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

