Thiadiazines, N,N-heterocycles of biological relevance
- PMID: 22732878
- PMCID: PMC6268759
- DOI: 10.3390/molecules17077612
Thiadiazines, N,N-heterocycles of biological relevance
Abstract
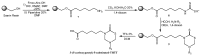
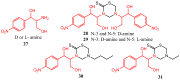
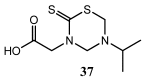
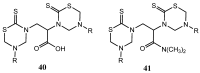
The 3,5-disubstituted tetrahydro-2H-1,3,5-thiadiazine-2-thione scaffold has found many applications in recent years. This review is aimed at highlighting the most important aspects of these compounds: synthesis, spectroscopic characterization and biological activities. How the chemical nature of N-substituents influences the overall activity and cytotoxicity profile will also be discussed.
Figures


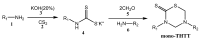
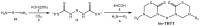
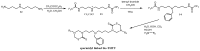











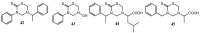
References
-
- Redtenbacher J., Liebig J. Ueber das Carbothildin. Liebigs Ann. Chem. 1848;65:43–45. doi: 10.1002/jlac.18480650104. - DOI
-
- Ainley A., Davies D. The constitution of the so-called carbothialdines and the preparation of some homologous compounds. J. Chem. Soc. 1944:147–149. doi: 10.1039/jr9440000147. - DOI
-
- Vogelsang H.D., Wagner-Jauregg T., Rebling R. Homologs of ethylene thiocyanohydrin and their ethers and thio ethers. Justus Liebigs Ann. Chem. 1950;569:183–198. doi: 10.1002/jlac.19505690303. - DOI
-
- Cummins E.W. 3,3'-Hydrocarbonylene bis(tetrahydro-1,3,5-thiadiazine-2-thiones) and fungicidal compositions. 3085046. US Patent. 1963 Apr 9;
-
- Martin D., Venker P. Preparation of S35-labeled carbon disulfide and 2-thio-3-benzyl-5-β-hydroxyethyltetrahydro-1,3,5-thiadiazine. Naturwissenschaften. 1962;49:256–257. doi: 10.1007/BF00601419. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

