Protection against Clostridium difficile infection with broadly neutralizing antitoxin monoclonal antibodies
- PMID: 22732923
- PMCID: PMC3491748
- DOI: 10.1093/infdis/jis416
Protection against Clostridium difficile infection with broadly neutralizing antitoxin monoclonal antibodies
Abstract
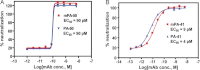
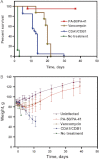
The spore-forming bacterium Clostridium difficile represents the principal cause of hospital-acquired diarrhea and pseudomembranous colitis worldwide. C. difficile infection (CDI) is mediated by 2 bacterial toxins, A and B; neutralizing these toxins with monoclonal antibodies (mAbs) provides a potential nonantibiotic strategy for combating the rising prevalence, severity, and recurrence of CDI. Novel antitoxin mAbs were generated in mice and were humanized. The humanized antitoxin A mAb PA-50 and antitoxin B mAb PA-41 have picomolar potencies in vitro and bind to novel regions of the respective toxins. In a hamster model for CDI, 95% of animals treated with a combination of humanized PA-50 and PA-41 showed long-term survival relative to 0% survival of animals treated with standard antibiotics or comparator mAbs. These humanized mAbs provide insight into C. difficile intoxication and hold promise as potential nonantibiotic agents for improving clinical management of CDI.
Figures

References
-
- Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36. - PubMed
-
- McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. - PubMed
-
- Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. - PubMed
-
- Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74:309–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

