Improved spatial targeting with directionally segmented deep brain stimulation leads for treating essential tremor
- PMID: 22732947
- PMCID: PMC3724530
- DOI: 10.1088/1741-2560/9/4/046005
Improved spatial targeting with directionally segmented deep brain stimulation leads for treating essential tremor
Abstract
Deep brain stimulation (DBS) in the ventral intermediate nucleus of thalamus (Vim) is known to exert a therapeutic effect on postural and kinetic tremor in patients with essential tremor (ET). For DBS leads implanted near the caudal border of Vim, however, there is an increased likelihood that one will also induce paresthesia side-effects by stimulating neurons within the sensory pathway of the ventral caudal (Vc) nucleus of thalamus. The aim of this computational study was to (1) investigate the neuronal pathways modulated by therapeutic, sub-therapeutic and paresthesia-inducing DBS settings in three patients with ET and (2) determine how much better an outcome could have been achieved had these patients been implanted with a DBS lead containing directionally segmented electrodes (dDBS). Multi-compartment neuron models of the thalamocortical, cerebellothalamic and medial lemniscal pathways were first simulated in the context of patient-specific anatomies, lead placements and programming parameters from three ET patients who had been implanted with Medtronic 3389 DBS leads. The models showed that in these patients, complete suppression of tremor was associated most closely with activating an average of 62% of the cerebellothalamic afferent input into Vim (n = 10), while persistent paresthesias were associated with activating 35% of the medial lemniscal tract input into Vc thalamus (n = 12). The dDBS lead design demonstrated superior targeting of the cerebello-thalamo-cortical pathway, especially in cases of misaligned DBS leads. Given the close proximity of Vim to Vc thalamus, the models suggest that dDBS will enable clinicians to more effectively sculpt current through and around thalamus in order to achieve a more consistent therapeutic effect without inducing side-effects.
Conflict of interest statement
Figures



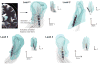


References
-
- Barbe MT, Liebhart L, Runge M, Deyng J, Florin E, Wojtecki L, Schnitzler A, Allert N, Sturm V, Fink GR, Maarouf M, Timmermann L. Deep brain stimulation of the ventral intermediate nucleus in patients with essential tremor: stimulation below intercommissural line is more efficient but equally effective as stimulation above. Experimental neurology. 2011a;230:131–137. - PubMed
-
- Barbe MT, Liebhart L, Runge M, Pauls KA, Wojtecki L, Schnitzler A, Allert N, Fink GR, Sturm V, Maarouf M, Timmermann L. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. Journal of neurology. 2011b;258:434–439. - PubMed
-
- Baron MS, Sidibe M, DeLong MR, Smith Y. Course of motor and associative pallidothalamic projections in monkeys. The Journal of comparative neurology. 2001;429:490–501. - PubMed
-
- Benabid AL, Lebas JF, Grand S, Benazzouz A, Pollak P, Krack P, Koudsie A, Chabardes S, Fraix V, Limousin P, Pinto S, Hoffmann D, Ardouin C, Funkiewiez A. Deep brain stimulation for movement disorders. In: Winn HR, editor. Youmans Neurological Surgery. Philadelphia: Saunders; 2004.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
