Accurate decoding of reaching movements from field potentials in the absence of spikes
- PMID: 22733013
- PMCID: PMC3429374
- DOI: 10.1088/1741-2560/9/4/046006
Accurate decoding of reaching movements from field potentials in the absence of spikes
Abstract
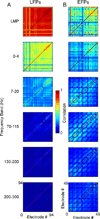
The recent explosion of interest in brain-machine interfaces (BMIs) has spurred research into choosing the optimal input signal source for a desired application. The signals with highest bandwidth--single neuron action potentials or spikes--typically are difficult to record for more than a few years after implantation of intracortical electrodes. Fortunately, field potentials recorded within the cortex (local field potentials, LFPs), at its surface (electrocorticograms, ECoG) and at the dural surface (epidural, EFPs) have also been shown to contain significant information about movement. However, the relative performance of these signals has not yet been directly compared. Furthermore, while it is widely postulated, it has not yet been demonstrated that these field potential signals are more durable than spike recordings. The aim of this study was to address both of these questions. We assessed the offline decoding performance of EFPs, LFPs and spikes, recorded sequentially, in primary motor cortex (M1) in terms of their ability to decode the target of reaching movements, as well as the endpoint trajectory. We also examined the decoding performance of LFPs on electrodes that are not recording spikes, compared with the performance when they did record spikes. Spikes were still present on some of the other electrodes throughout this study. We showed that LFPs performed nearly as well as spikes in decoding velocity, and slightly worse in decoding position and in target classification. EFP performance was slightly inferior to that reported for ECoG in humans. We also provided evidence demonstrating that movement-related information in the LFP remains high regardless of the ability to record spikes concurrently on the same electrodes. This is the first study to provide evidence that LFPs retain information about movement in the absence of spikes on the same electrodes. These results suggest that LFPs may indeed remain informative after spike recordings are lost, thereby providing a robust, accurate signal source for BMIs.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures







Similar articles
-
Reliability of directional information in unsorted spikes and local field potentials recorded in human motor cortex.J Neural Eng. 2014 Aug;11(4):046007. doi: 10.1088/1741-2560/11/4/046007. Epub 2014 Jun 12. J Neural Eng. 2014. PMID: 24921388 Free PMC article.
-
The utility of multichannel local field potentials for brain-machine interfaces.J Neural Eng. 2013 Aug;10(4):046005. doi: 10.1088/1741-2560/10/4/046005. Epub 2013 Jun 7. J Neural Eng. 2013. PMID: 23744624 Free PMC article.
-
Decoding 3D reach and grasp from hybrid signals in motor and premotor cortices: spikes, multiunit activity, and local field potentials.J Neurophysiol. 2012 Mar;107(5):1337-55. doi: 10.1152/jn.00781.2011. Epub 2011 Dec 7. J Neurophysiol. 2012. PMID: 22157115 Free PMC article.
-
Decoding of attentional state using local field potentials.Curr Opin Neurobiol. 2022 Oct;76:102589. doi: 10.1016/j.conb.2022.102589. Epub 2022 Jun 22. Curr Opin Neurobiol. 2022. PMID: 35751949 Free PMC article. Review.
-
Physiological properties of brain-machine interface input signals.J Neurophysiol. 2017 Aug 1;118(2):1329-1343. doi: 10.1152/jn.00070.2017. Epub 2017 Jun 14. J Neurophysiol. 2017. PMID: 28615329 Free PMC article. Review.
Cited by
-
Reliability of directional information in unsorted spikes and local field potentials recorded in human motor cortex.J Neural Eng. 2014 Aug;11(4):046007. doi: 10.1088/1741-2560/11/4/046007. Epub 2014 Jun 12. J Neural Eng. 2014. PMID: 24921388 Free PMC article.
-
Decoding continuous limb movements from high-density epidural electrode arrays using custom spatial filters.J Neural Eng. 2013 Jun;10(3):036015. doi: 10.1088/1741-2560/10/3/036015. Epub 2013 Apr 23. J Neural Eng. 2013. PMID: 23611833 Free PMC article.
-
Multimodal decoding and congruent sensory information enhance reaching performance in subjects with cervical spinal cord injury.Front Neurosci. 2014 May 23;8:123. doi: 10.3389/fnins.2014.00123. eCollection 2014. Front Neurosci. 2014. PMID: 24904265 Free PMC article.
-
Nucleotide-time alignment for molecular recorders.PLoS Comput Biol. 2017 May 1;13(5):e1005483. doi: 10.1371/journal.pcbi.1005483. eCollection 2017 May. PLoS Comput Biol. 2017. PMID: 28459860 Free PMC article.
-
Decoding finger movement in humans using synergy of EEG cortical current signals.Sci Rep. 2017 Sep 12;7(1):11382. doi: 10.1038/s41598-017-09770-5. Sci Rep. 2017. PMID: 28900188 Free PMC article.
References
-
- Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416:141–142. - PubMed
-
- Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–1832. - PubMed
-
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. - PubMed
-
- Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
