Interleukin-10 spot-forming cells as a novel biomarker of chronic graft-versus-host disease
- PMID: 22733028
- PMCID: PMC3533658
- DOI: 10.3324/haematol.2012.069815
Interleukin-10 spot-forming cells as a novel biomarker of chronic graft-versus-host disease
Abstract
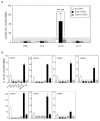
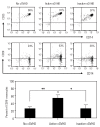
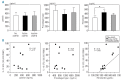
Although there are National Institutes of Health consensus criteria for the global assessment of chronic graft-versus-host disease, no validated biomarkers have been established for this disease. Furthermore, whereas the role of T cells, B cells, and dendritic cells in chronic graft-versus-host disease has been established, the contribution of monocytes has not been clearly addressed. Using an enzyme-linked immunospot assay, we measured the spot-forming cells for interferon-γ, interleukin-4, interleukin-10, and interleukin-17 in unstimulated peripheral blood of patients following allogeneic hematopoietic stem cell transplantation. Other immunological examinations, including skin biopsy, were also done. Fifty-seven patients were enrolled. Interleukin-10 spot-forming cells were evaluable for therapeutic monitoring in 16 patients with chronic graft-versus-host disease. The number of interleukin-10 spot-forming cells in patients with active chronic graft-versus-host disease was significantly higher than the number in those with no or inactive chronic graft-versus-host disease. Interleukin-10 was predominantly produced by monocytes. CD29 expression on monocytes in patients with active chronic graft-versus-host disease was elevated. The level of plasma fibronectin, a ligand of CD29, correlated with the number of interleukin-10 spot-forming cells. Immunohistochemical analysis of the skin in active chronic graft-versus-host disease showed that infiltrating CD29(+) monocytes might produce interleukin-10. A novel biomarker, interleukin-10 spot-forming cells, shows promise as both a diagnostic and prognostic indicator for chronic graft-versus-host disease, and may allow for early intervention prior to the onset of the disease. Measurement of interleukin-10 spot-forming cells would be helpful in clinical trials and in patients' management.
Figures


References
-
- Pidala J, Kim J, Anasetti C, Nishihori T, Betts B, Field T, et al. The global severity of chronic graft-versus-host disease, determined by National Institutes of Health consensus criteria, is associated with overall survival and non-relapse mortality. Haematologica. 2011;96(11):1678-84 - PMC - PubMed
-
- Socie G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101(9):3373-85 - PubMed
-
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945-56 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

