Antiviral drug resistance and the need for development of new HIV-1 reverse transcriptase inhibitors
- PMID: 22733071
- PMCID: PMC3457356
- DOI: 10.1128/AAC.00591-12
Antiviral drug resistance and the need for development of new HIV-1 reverse transcriptase inhibitors
Abstract
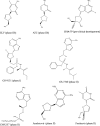
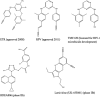
Highly active antiretroviral therapy (HAART) consists of a combination of drugs to achieve maximal virological response and reduce the potential for the emergence of antiviral resistance. Despite being the first antivirals described to be effective against HIV, reverse transcriptase inhibitors remain the cornerstone of HAART. There are two broad classes of reverse transcriptase inhibitor, the nucleoside reverse transcriptase inhibitors (NRTIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs). Since the first such compounds were developed, viral resistance to them has inevitably been described; this necessitates the continuous development of novel compounds within each class. In this review, we consider the NRTIs and NNRTIs currently in both preclinical and clinical development or approved for second-line therapy and describe the patterns of resistance associated with their use as well as the underlying mechanisms that have been described. Due to reasons of both affordability and availability, some reverse transcriptase inhibitors with a low genetic barrier are more commonly used in resource-limited settings. Their use results in the emergence of specific patterns of antiviral resistance and so may require specific actions to preserve therapeutic options for patients in such settings.
Figures
References
-
- Antinori A, et al. 2002. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res. Hum. Retroviruses 18:835–838 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical