MBX-500, a hybrid antibiotic with in vitro and in vivo efficacy against toxigenic Clostridium difficile
- PMID: 22733075
- PMCID: PMC3421853
- DOI: 10.1128/AAC.00508-12
MBX-500, a hybrid antibiotic with in vitro and in vivo efficacy against toxigenic Clostridium difficile
Abstract
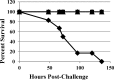
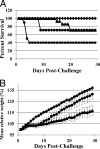
Clostridium difficile infection (CDI) causes moderate to severe disease, resulting in diarrhea and pseudomembranous colitis. CDI is difficult to treat due to production of inflammation-inducing toxins, resistance development, and high probability of recurrence. Only two antibiotics are approved for the treatment of CDI, and the pipeline for therapeutic agents contains few new drugs. MBX-500 is a hybrid antibacterial, composed of an anilinouracil DNA polymerase inhibitor linked to a fluoroquinolone DNA gyrase/topoisomerase inhibitor, with potential as a new therapeutic for CDI treatment. Since MBX-500 inhibits three bacterial targets, it has been previously shown to be minimally susceptible to resistance development. In the present study, the in vitro and in vivo efficacies of MBX-500 were explored against the Gram-positive anaerobe, C. difficile. MBX-500 displayed potency across nearly 50 isolates, including those of the fluoroquinolone-resistant, toxin-overproducing NAP1/027 ribotype, performing as well as comparator antibiotics vancomycin and metronidazole. Furthermore, MBX-500 was a narrow-spectrum agent, displaying poor activity against many other gut anaerobes. MBX-500 was active in acute and recurrent infections in a toxigenic hamster model of CDI, exhibiting full protection against acute infections and prevention of recurrence in 70% of the animals. Hamsters treated with MBX-500 displayed significantly greater weight gain than did those treated with vancomycin. Finally, MBX-500 was efficacious in a murine model of CDI, again demonstrating a fully protective effect and permitting near-normal weight gain in the treated animals. These selective anti-CDI features support the further development of MBX 500 for the treatment of CDI.
Figures

References
-
- Aas J, Gessert CE, Bakken JS. 2003. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin. Infect. Dis. 36:580–585 - PubMed
-
- Allen SD, Emery CL, Siders JA. 1999. Clostridium, p 654–671 In Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. (ed), Manual of clinical microbiology, 7th ed ASM Press, Washington, DC
-
- Bartlett JG. 2006. New drugs for Clostridium difficile infection. Clin. Infect. Dis. 43:428–431 - PubMed
-
- Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 298:531–534 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

