Differential effects of a soluble or immobilized VEGFR-binding peptide
- PMID: 22733256
- PMCID: PMC3415255
- DOI: 10.1039/c2ib20055d
Differential effects of a soluble or immobilized VEGFR-binding peptide
Abstract
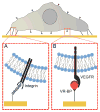
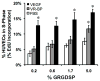
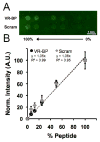
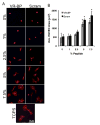
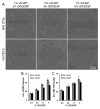
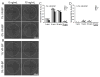
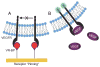
Regulating endothelial cell behavior is a key step in understanding and controlling neovascularization for both pro-angiogenic and anti-angiogenic therapeutic strategies. Here, we characterized the effects of a covalently immobilized peptide mimic of vascular endothelial growth factor, herein referred to as VEGF receptor-binding peptide (VR-BP), on human umbilical vein endothelial cell (HUVEC) behavior. Self-assembled monolayer arrays presenting varied densities of covalently immobilized VR-BP and varied densities of the fibronectin-derived cell adhesion peptide Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP) were used to probe for changes in HUVEC attachment, proliferation and tubulogenesis. In a soluble form, VR-BP exhibited pro-angiogenic effects in agreement with previous studies, indicated by increases in HUVEC proliferation. However, when presented to cells in an insoluble context, covalently immobilized VR-BP inhibited several pro-angiogenic HUVEC behaviors, including attachment and proliferation, and also inhibited HUVEC response to soluble recombinant VEGF protein. Furthermore, substrates with covalently immobilized VR-BP also modulated HUVEC tubulogenesis when a matrigel overlay assay was used to provide cells with a pseudo-three dimensional environment. Taken together, these results demonstrate that the context in which ligands are presented to cell surface receptors strongly influences their effects, and that the same ligand can be an agonist or an antagonist depending on the manner of presentation to the cell.
Figures


References
-
- Cross MJ, et al. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28(9):488–94. - PubMed
-
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. - PubMed
-
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–86. - PubMed
-
- D’Andrea LD, et al. Peptide-based molecules in angiogenesis. Chem Biol Drug Des. 2006;67(2):115–26. - PubMed
-
- Soker S, et al. Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation by a peptide corresponding to the exon 7-encoded domain of VEGF165. J Biol Chem. 1997;272(50):31582–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

