Mechanosensitivity of nicotinic receptors
- PMID: 22733356
- PMCID: PMC3395360
- DOI: 10.1007/s00424-012-1132-9
Mechanosensitivity of nicotinic receptors
Abstract
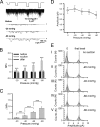
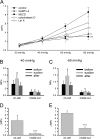
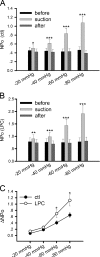
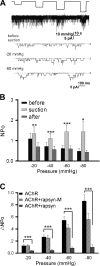
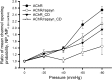
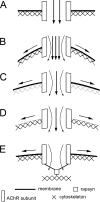
Nicotinic acetylcholine receptors (nAChRs) are heteropentameric ligand-gated ion channels that mediate excitatory neurotransmission at the neuromuscular junction (NMJ) and other peripheral and central synapses. At the NMJ, acetylcholine receptors (AChRs) are constantly exposed to mechanical stress resulting from muscle contraction. It is therefore of interest to understand if their function is influenced by mechanical stimuli. In this study, patch-clamp recordings showed that AChR channel activity was enhanced upon membrane stretching in both cultured Xenopus muscle cells and C2C12 myotubes. To examine how this property is physiologically regulated, effects of membrane-intrinsic and membrane-extrinsic factors on AChRs expressed in HEK293T cells were studied. As in muscle cells, AChR single channel currents recorded under cell-attached configuration were significantly increased-without change in current amplitude-when negative pressure was applied through the patch pipette. GsMTx-4, a peptide toxin that blocks mechanically activated cation channels, inhibited this effect on AChRs. The mechanosensitivity decreased when cells were treated with MβCD, latrunculin A or cytochalasin D, but increased when exposed to lysophosphatidylcholine, indicating contributions from both membrane lipids and the cytoskeleton. Rapsyn, which binds to AChRs and mediates their cytoskeletal interaction in muscle, suppressed AChR mechanosensitivity when co-expressed in HEK293T cells, but this influence of rapsyn was impaired following the deletion of rapsyn's AChR-binding domain or upon cytoskeletal disruption by cytochalasin D. These results suggest a mechanism for regulating AChR's mechanosensitivity through its cytoskeletal linkage via rapsyn, which may serve to protect the receptors and sarcolemmal integrity under high mechanical stress encountered by the NMJ.
Figures

Similar articles
-
Fast desensitization of acetylcholine receptors induced by a spider toxin.Channels (Austin). 2021 Dec;15(1):507-515. doi: 10.1080/19336950.2021.1961459. Channels (Austin). 2021. PMID: 34374321 Free PMC article.
-
Rapsyn facilitates recovery from desensitization in fetal and adult acetylcholine receptors expressed in a muscle cell line.J Physiol. 2019 Jul;597(14):3713-3725. doi: 10.1113/JP277819. Epub 2019 Jun 17. J Physiol. 2019. PMID: 31158924 Free PMC article.
-
Src-family kinases stabilize the neuromuscular synapse in vivo via protein interactions, phosphorylation, and cytoskeletal linkage of acetylcholine receptors.J Neurosci. 2005 Nov 9;25(45):10479-93. doi: 10.1523/JNEUROSCI.2103-05.2005. J Neurosci. 2005. PMID: 16280586 Free PMC article.
-
Rapsyn as a signaling and scaffolding molecule in neuromuscular junction formation and maintenance.Neurosci Lett. 2020 Jul 13;731:135013. doi: 10.1016/j.neulet.2020.135013. Epub 2020 Apr 26. Neurosci Lett. 2020. PMID: 32344108 Review.
-
Structural basis for lipid modulation of nicotinic acetylcholine receptor function.Brain Res Brain Res Rev. 2004 Dec;47(1-3):71-95. doi: 10.1016/j.brainresrev.2004.06.008. Brain Res Brain Res Rev. 2004. PMID: 15572164 Review.
Cited by
-
Cardiac Mechano-Gated Ion Channels and Arrhythmias.Circ Res. 2016 Jan 22;118(2):311-29. doi: 10.1161/CIRCRESAHA.115.305043. Circ Res. 2016. PMID: 26838316 Free PMC article. Review.
-
Stretching and setting boundaries.J Gen Physiol. 2012 Oct;140(4):341-2. doi: 10.1085/jgp.201210895. J Gen Physiol. 2012. PMID: 23008431 Free PMC article. No abstract available.
-
Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1.Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):E1162-8. doi: 10.1073/pnas.1219777110. Epub 2013 Mar 4. Proc Natl Acad Sci U S A. 2013. PMID: 23487776 Free PMC article.
-
Fast desensitization of acetylcholine receptors induced by a spider toxin.Channels (Austin). 2021 Dec;15(1):507-515. doi: 10.1080/19336950.2021.1961459. Channels (Austin). 2021. PMID: 34374321 Free PMC article.
-
The nematode C. elegans senses airborne sound.Neuron. 2021 Nov 17;109(22):3633-3646.e7. doi: 10.1016/j.neuron.2021.08.035. Epub 2021 Sep 22. Neuron. 2021. PMID: 34555314 Free PMC article.
References
-
- Ballard RE, Watenpaugh DE, Breit GA, Murthy G, Holley DC, Hargens AR. Leg intramuscular pressures during locomotion in humans. J Appl Physiol. 1998;84:1976–1981. - PubMed
-
- Borges LS, Yechikhov S, Lee YI, Rudell JB, Friese MB, Burden SJ, Ferns MJ. Identification of a motif in the acetylcholine receptor beta subunit whose phosphorylation regulates rapsyn association and postsynaptic receptor localization. J Neurosci. 2008;28:11468–11476. doi: 10.1523/JNEUROSCI.2508-08.2008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

