The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations
- PMID: 22733536
- PMCID: PMC3682221
- DOI: 10.1158/1078-0432.CCR-12-0357
The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations
Abstract
Purpose: This retrospective study was undertaken to investigate the impact of initial gefitinib or erlotinib (EGFR tyrosine kinase inhibitor, EGFR-TKI) versus chemotherapy on the risk of central nervous system (CNS) progression in advanced non-small cell lung cancer (NSCLC) with EGFR mutations.
Experimental design: Patients with stage IV or relapsed NSCLC with a sensitizing EGFR mutation initially treated with gefitinib, erlotinib, or chemotherapy were identified. The cumulative risk of CNS progression was calculated using death as a competing risk.
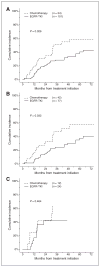
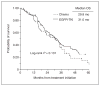
Results: One hundred and fifty-five patients were eligible (EGFR-TKI: 101, chemotherapy: 54). Twenty-four patients (24%) in the EGFR-TKI group and 12 patients (22%) in the chemotherapy group had brain metastases at the time of diagnosis of advanced NSCLC (P = 1.000); 32 of the 36 received CNS therapy before initiating systemic treatment. Thirty-three patients (33%) in the EGFR-TKI group and 26 patients (48%) in the chemotherapy group developed CNS progression after a median follow-up of 25 months. The 6-, 12-, and 24-month cumulative risk of CNS progression was 1%, 6%, and 21% in the EGFR-TKI group compared with corresponding rates of 7%, 19%, and 32% in the chemotherapy group (P = 0.026). The HR of CNS progression for upfront EGFR-TKI versus chemotherapy was 0.56 [95% confidence interval (CI), 0.34-0.94].
Conclusions: Our data show lower rates of CNS progression in EGFR-mutant advanced NSCLC patients initially treated with an EGFR-TKI compared with upfront chemotherapy. If validated, our results suggest that gefitinib and erlotinib may have a role in the chemoprevention of CNS metastases from NSCLC.
Conflict of interest statement
V.A. Joshi: employment (KEW Group); ownership interest (KEW Group). D.B. Costa: consultant/advisory board (Pfizer; AstraZeneca; Roche). M.S. Rabin: consultant/advisory board (Genentech). D.M. Jackman: consultant/advisory board (Foundation Medicine; Genentech). B.E. Johnson: ownership interest (KEW Group); consultant/advisory board (Genentech; Pfizer; Chugai; AstraZeneca); post marketing royalties for EGFR mutation testing. No potential conflicts of interest were disclosed by the other authors.
Figures
References
-
- Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23:6207–19. - PubMed
-
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. - PubMed
-
- Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–91. - PubMed
-
- Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: clinical implications for the subsequent management of the brain. Cancer. 2007;109:1668–75. - PubMed
-
- Mamon HJ, Yeap BY, Janne PA, Reblando J, Shrager S, Jaklitsch MT, et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol. 2005;23:1530–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous