On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves
- PMID: 22733731
- PMCID: PMC3396524
- DOI: 10.1073/pnas.1209288109
On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves
Abstract
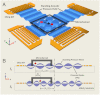
Techniques that can dexterously manipulate single particles, cells, and organisms are invaluable for many applications in biology, chemistry, engineering, and physics. Here, we demonstrate standing surface acoustic wave based "acoustic tweezers" that can trap and manipulate single microparticles, cells, and entire organisms (i.e., Caenorhabditis elegans) in a single-layer microfluidic chip. Our acoustic tweezers utilize the wide resonance band of chirped interdigital transducers to achieve real-time control of a standing surface acoustic wave field, which enables flexible manipulation of most known microparticles. The power density required by our acoustic device is significantly lower than its optical counterparts (10,000,000 times less than optical tweezers and 100 times less than optoelectronic tweezers), which renders the technique more biocompatible and amenable to miniaturization. Cell-viability tests were conducted to verify the tweezers' compatibility with biological objects. With its advantages in biocompatibility, miniaturization, and versatility, the acoustic tweezers presented here will become a powerful tool for many disciplines of science and engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Moved by sound.Nat Methods. 2012 Sep;9(9):867. doi: 10.1038/nmeth.2150. Nat Methods. 2012. PMID: 23097785 No abstract available.
References
-
- Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–731. - PubMed
-
- Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt Lett. 1986;11:288–290. - PubMed
-
- Grier DG. A revolution in optical manipulation. Nature. 2003;424:810–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

