Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes
- PMID: 22733772
- PMCID: PMC3396532
- DOI: 10.1073/pnas.1208708109
Acute augmentation of epoxygenated fatty acid levels rapidly reduces pain-related behavior in a rat model of type I diabetes
Abstract
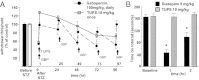
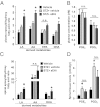
The nerve damage occurring as a consequence of glucose toxicity in diabetes leads to neuropathic pain, among other problems. This pain dramatically reduces the quality of life in afflicted patients. The progressive damage to the peripheral nervous system is irreversible although strict control of hyperglycemia may prevent further damage. Current treatments include tricyclic antidepressants, anticonvulsants, and opioids, depending on the severity of the pain state. However, available therapeutics have drawbacks, arguing for the need to better understand the pathophysiology of neuropathic pain and develop novel treatments. Here we demonstrate that stabilization of a class of bioactive lipids, epoxygenated fatty acids (EpFAs), greatly reduces allodynia in rats caused by streptozocin-induced type I diabetes. Inhibitors of the soluble epoxide hydrolase (sEHI) elevated and stabilized the levels of plasma and spinal EpFAs, respectively, and generated dose-dependent antiallodynic effects more potently and efficaciously than gabapentin. In acute experiments, positive modulation of EpFAs did not display differences in insulin sensitivity, glucose tolerance, or insulin secretion, indicating the efficacy of sEHIs are not related to the glycemic status. Quantitative metabolomic analysis of a panel of 26 bioactive lipids demonstrated that sEHI-mediated antiallodynic effects coincided with a selective elevation of the levels of EpFAs in the plasma, and a decrease in degradation products coincided with the dihydroxy fatty acids in the spinal cord. Overall, these results argue that further efforts in understanding the spectrum of effects of EpFAs will yield novel opportunities in treating neuropathic pain.
Conflict of interest statement
Conflict of interest statement. B.I., K.M.W., S.H.H., C.M., and B.D.H are coinventors on patents related to inhibitors of the soluble epoxide hydrolase and pain by the University of California.
Figures

References
-
- Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68:928–930, 932, 934–944. - PubMed
-
- Said G. Diabetic neuropathy—A review. Nat Clin Pract Neurol. 2007;3:331–340. - PubMed
-
- Sima AAF, et al. Regeneration and repair of myelinated fibers in sural-nerve biopsy specimens from patients with diabetic neuropathy treated with sorbinil. N Engl J Med. 1988;319:548–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

